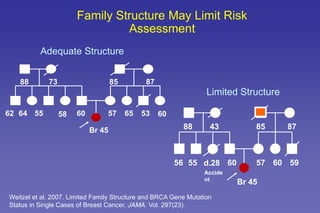
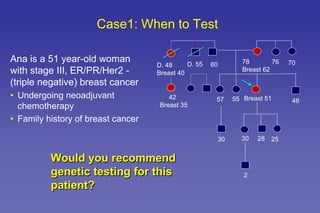
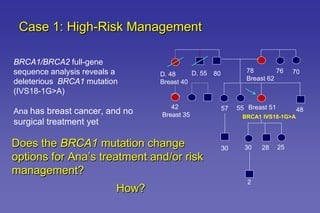
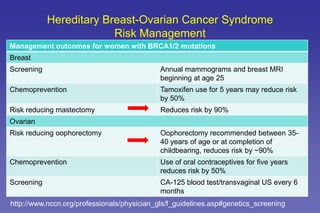
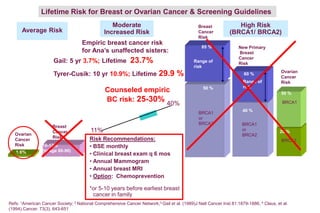
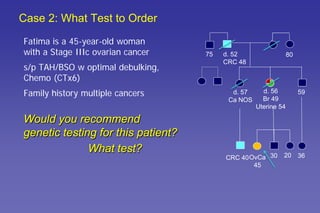
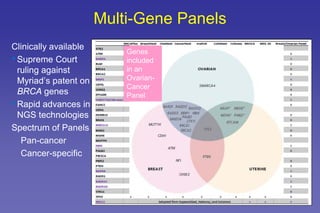
This document summarizes a presentation on genetic testing for cancer risk. The presentation covered:
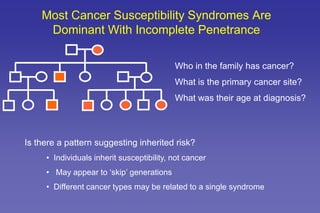
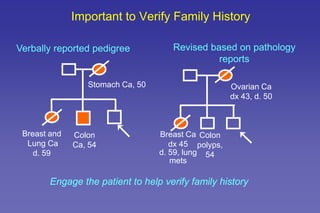
- The role of genetic cancer risk assessment in identifying inherited cancer risk.
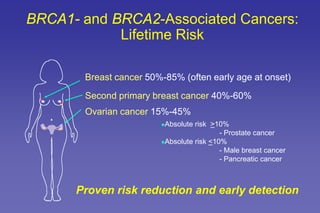
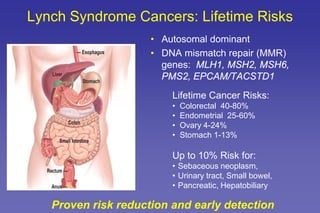
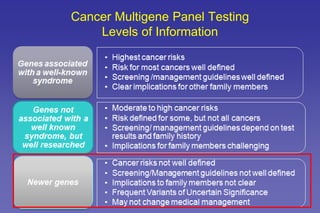
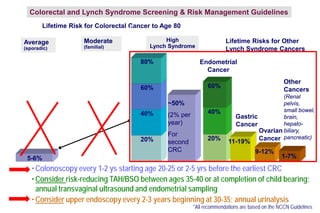
- Common hereditary cancer syndromes like BRCA1/2 and Lynch syndrome and their associated cancer risks.
- Guidelines for cancer screening and management based on genetic test results.
- The benefits and limitations of multi-gene cancer panels.
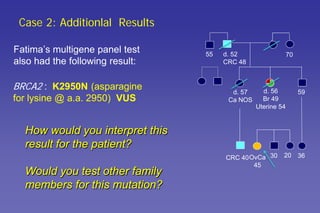
- How to interpret genetic test results and provide appropriate risk management recommendations to patients.