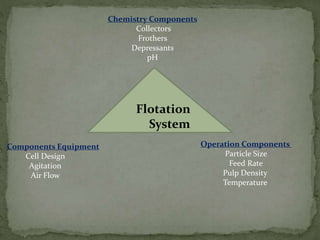
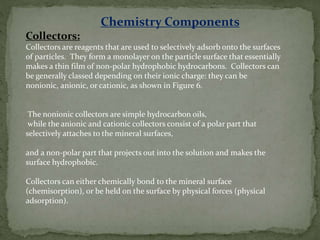
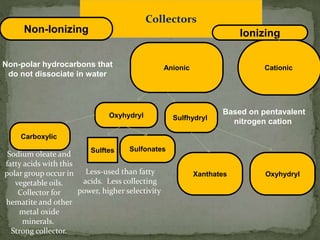
Froth flotation is a process that separates minerals based on their hydrophobicity. Hydrophobic particles attach to air bubbles introduced into the mineral slurry and float to the surface, where they can be removed. Hydrophilic particles remain suspended in the liquid. It is useful for processing fine-grained ores not amenable to gravity concentration. The process relies on the difference in wettability between minerals, with some easily wetted by water while others are water-repellent. Collectors are chemicals that are selectively adsorbed onto mineral surfaces to make them hydrophobic.