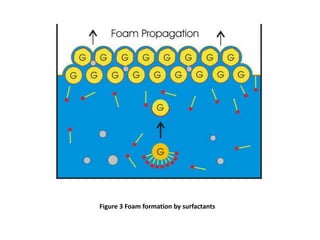
The document discusses froth and foam formation in amine treating processes. Froth is produced when gas is bubbled violently through an amine solution, creating mass transfer area. Froth turns to foam when the gas/liquid disengagement time increases, such as when surfactants are present which stabilize the bubble interface. Surfactants can enter from amine degradation or in feed/makeup streams. Higher surfactant concentrations, solution properties like viscosity and strength, and solids content all favor foam formation over froth. Antifoams are used to combat foaming but their effectiveness depends on factors like concentration and environmental conditions.