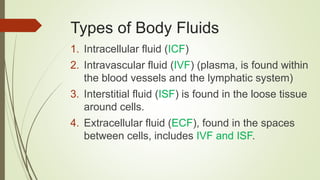
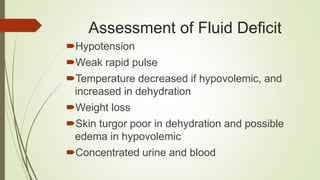
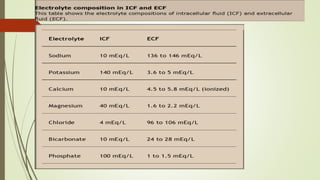
The document outlines the importance of fluids, electrolytes, and acid-base balance in human physiology, detailing the types of body fluids and their functions. It emphasizes the role of electrolytes in maintaining homeostasis and describes the mechanisms of fluid and electrolyte movement. Additionally, it covers acid-base balance regulation through various physiological systems and defines the effects of imbalances on health.