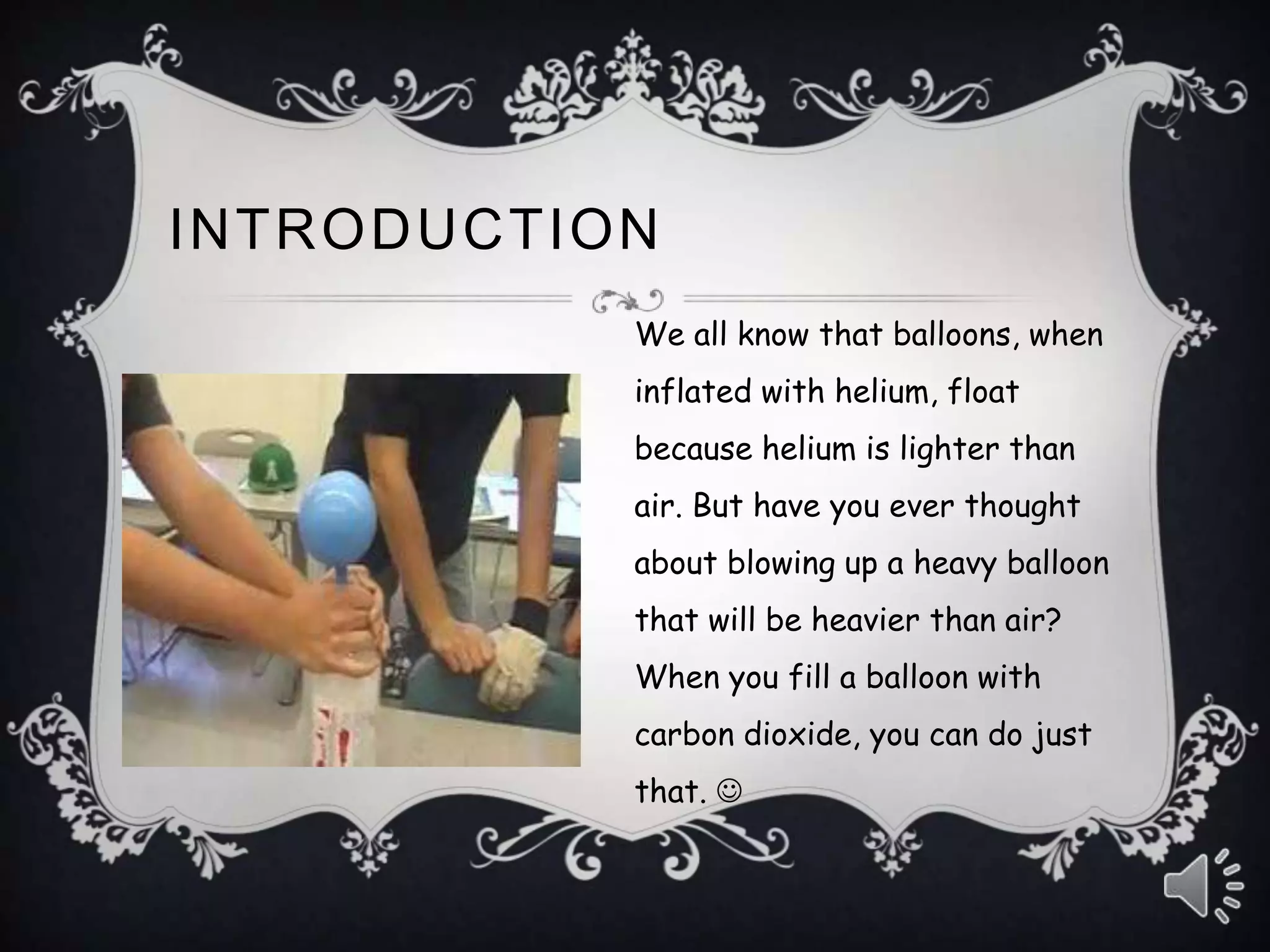
This document provides instructions for an experiment to make a heavy balloon filled with carbon dioxide gas. Baking soda and vinegar are mixed, which produce carbon dioxide gas that fills a balloon. The balloon filled with carbon dioxide is expected to fall faster than a normal air-filled balloon when dropped from the same height, since carbon dioxide is heavier than air. The experiment is carried out and observations confirm that the carbon dioxide balloon does fall faster, supporting the initial hypothesis.