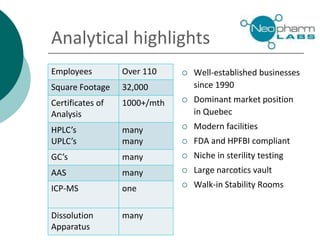
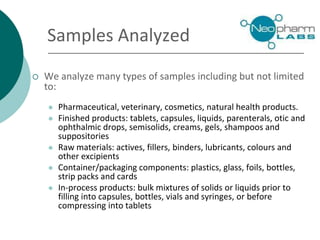
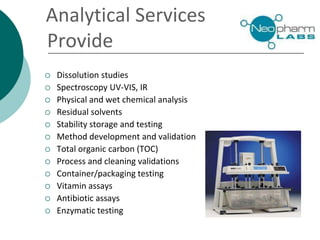
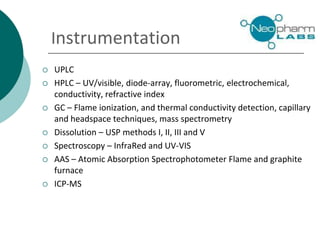
Neopharm Labs provides analytical testing services including chemistry, microbiology, sterility testing, and method development and validation to the pharmaceutical, biotech, cosmetic, and veterinary industries. It has over 110 employees and 32,000 square feet of facilities in Quebec, Canada. Neopharm is certified by the FDA and Health Canada and audited by global pharmaceutical companies. It has a wide range of instrumentation and offers services such as dissolution testing, stability studies, and microbiological assays.