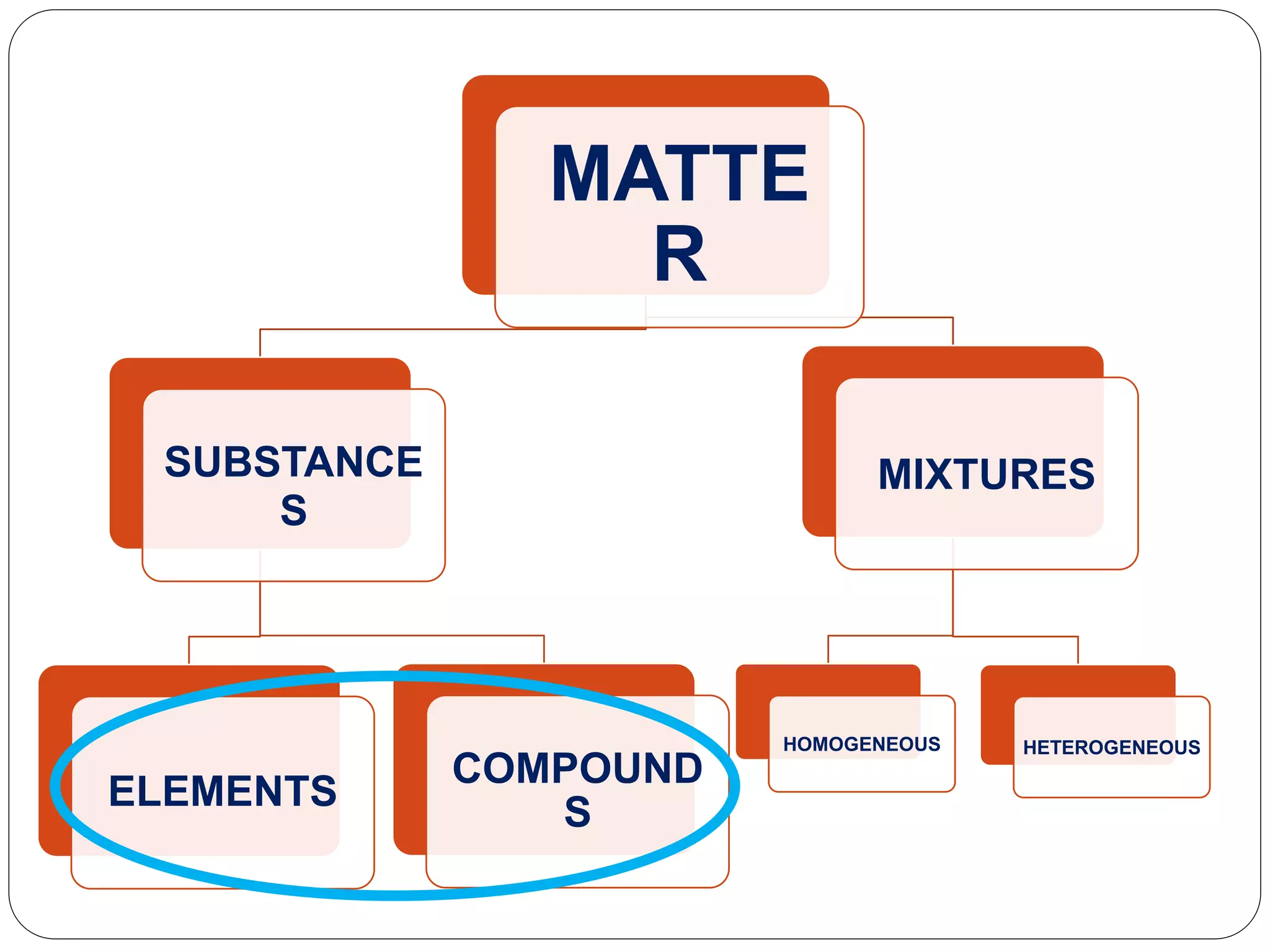
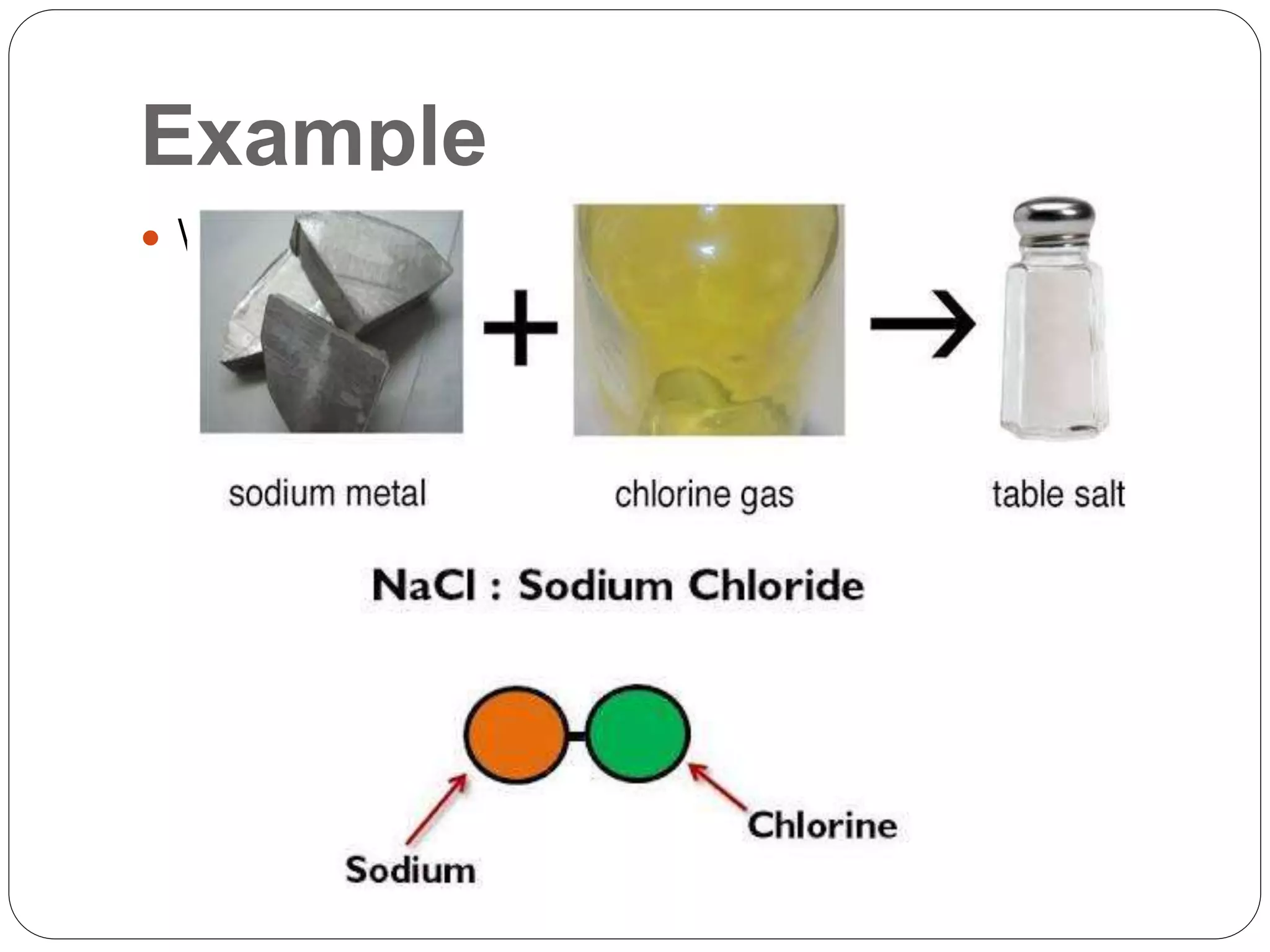
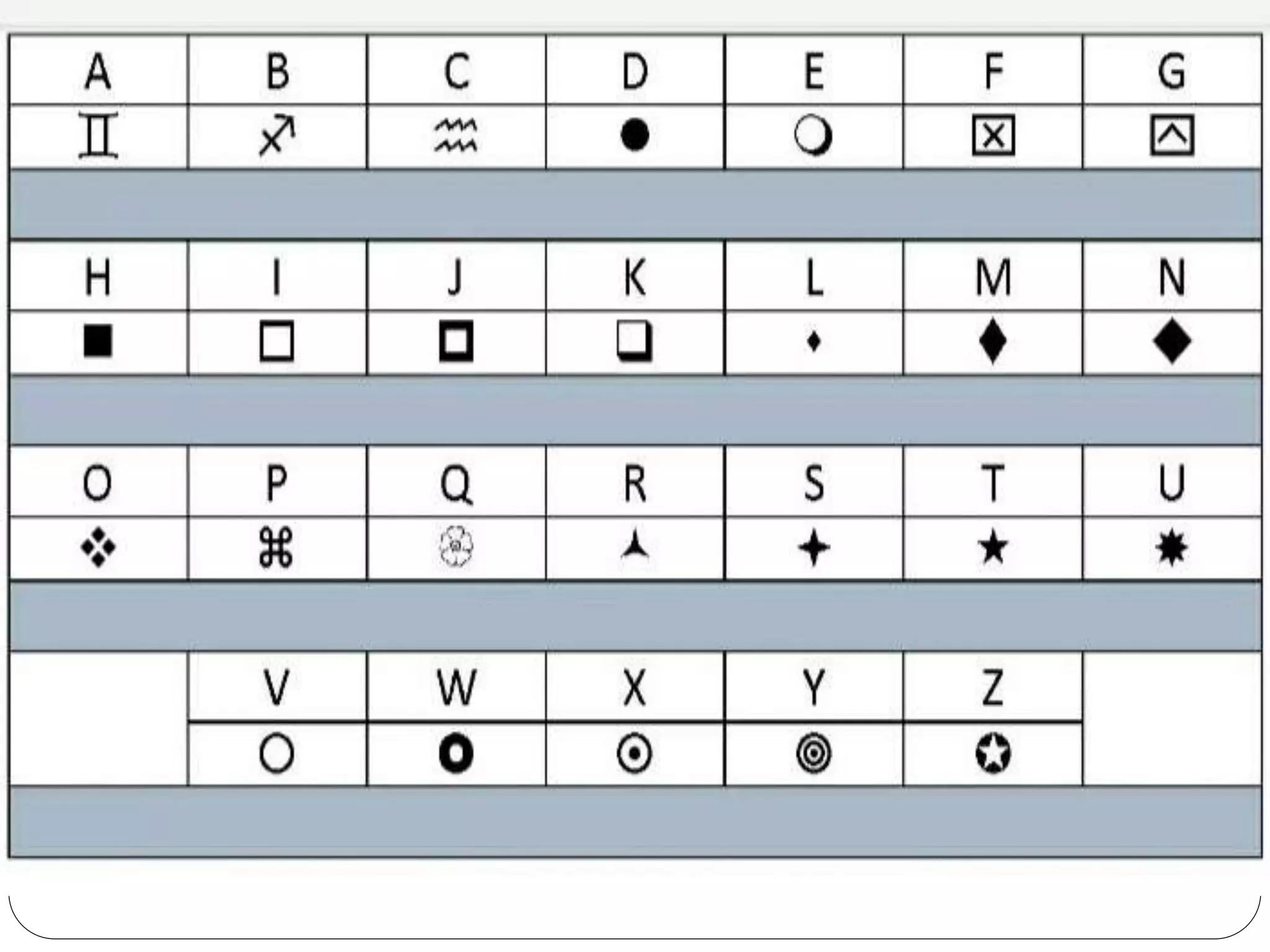
The document discusses the differences between substances and mixtures. Substances are homogeneous, meaning their composition is uniform throughout, while some mixtures are also homogeneous. Pure water is given as an example of a substance, while water mixed with salt is provided as an example of a homogeneous mixture. The key differences presented are that substances are uniform in composition while mixtures may have non-uniform compositions.