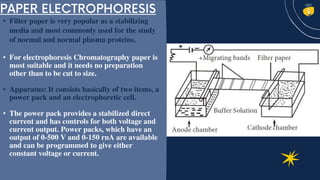
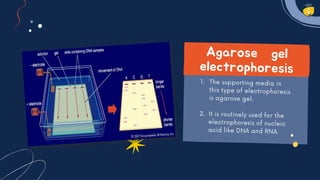
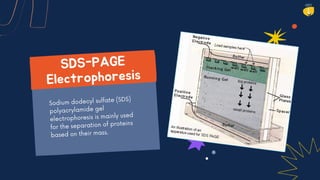
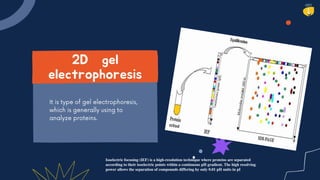
Electrophoresis is a technique used to separate charged molecules like proteins, nucleic acids, and other biomolecules. There are several types including free solution electrophoresis, zone electrophoresis using supporting media like paper, cellulose acetate, capillary, or gel electrophoresis. Gel electrophoresis is commonly used and separates biomolecules based on their size and charge as they migrate through a gel under an electric field. Electrophoresis has various applications including DNA analysis, studying protein interactions, and testing antibiotics and vaccines.