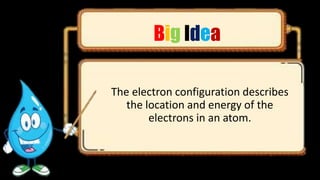
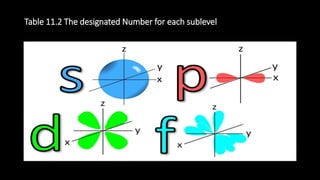
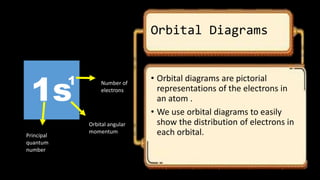
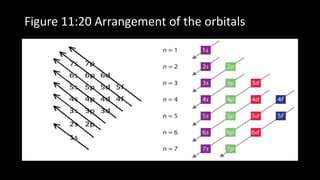
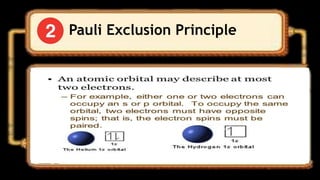
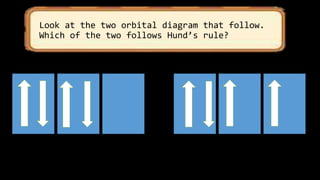
This document discusses the electron configuration of atoms. It begins by introducing the concept of electron configuration and how it describes the distribution of electrons in different energy levels and orbitals within an atom. It then defines the key terms used to describe electron configuration, including principal quantum number, angular momentum quantum number, magnetic quantum number, and orbital diagrams. Finally, it explains the three main rules that govern how electrons fill the orbitals - the Aufbau principle, Pauli exclusion principle, and Hund's rule.