Embed presentation
Download to read offline
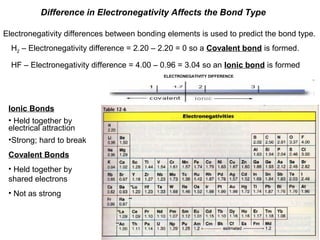

The document discusses how differences in electronegativity between bonding elements determine the type of bond formed. For instance, in H2, a covalent bond forms due to an electronegativity difference of 0, while in HF, an ionic bond forms with a difference of 3.04. It further describes the characteristics of ionic bonds as strong and difficult to break, compared to covalent bonds, which are held together by shared electrons and are relatively weaker.
