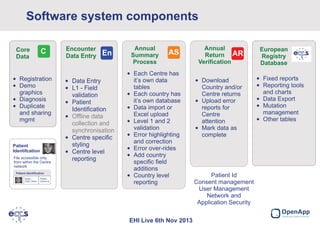
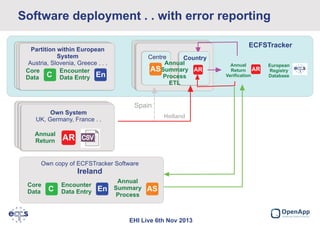
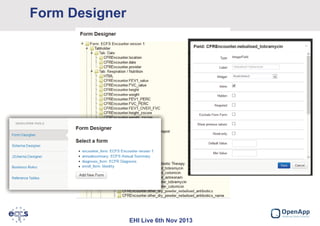
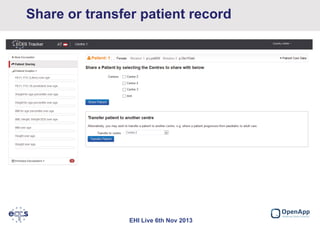
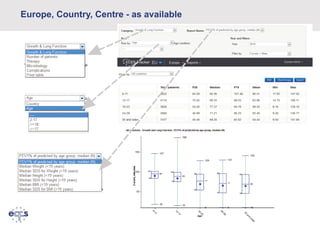
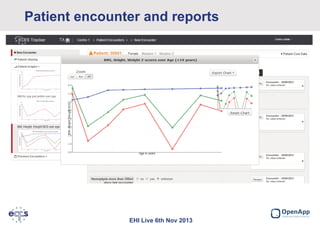
This document summarizes a presentation about the European Cystic Fibrosis Patient Registry (ECFSPR). The ECFSPR collects data from over 23 countries and 220 hospitals/centers to measure and compare aspects of cystic fibrosis treatment. It aims to encourage new treatment standards and provide data for research. The presentation discusses the OpenApp software developed for the registry, which allows different countries and centers to participate through a shared or customized system while protecting patient data. Features like error reporting and flexible reporting are highlighted.