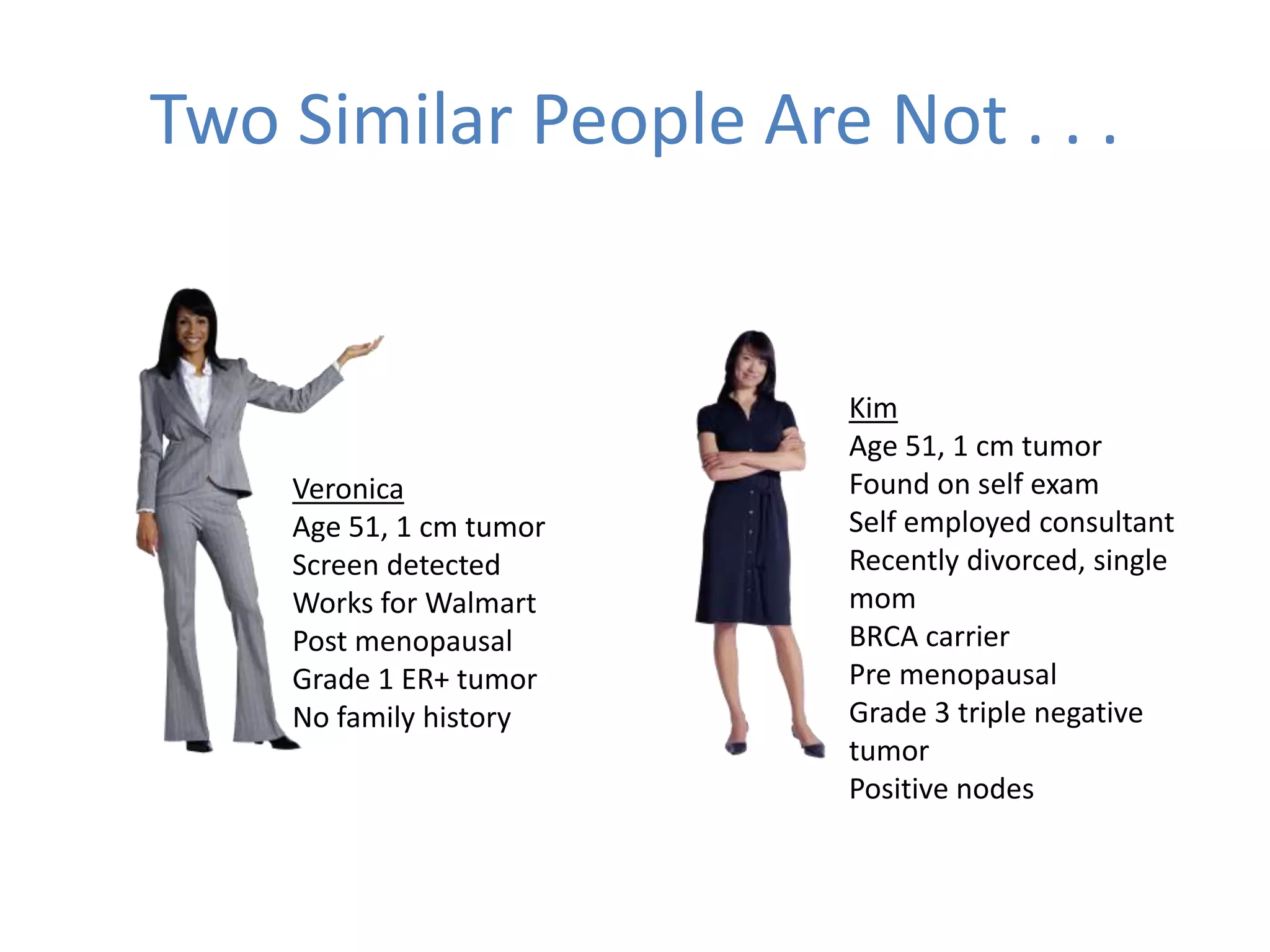
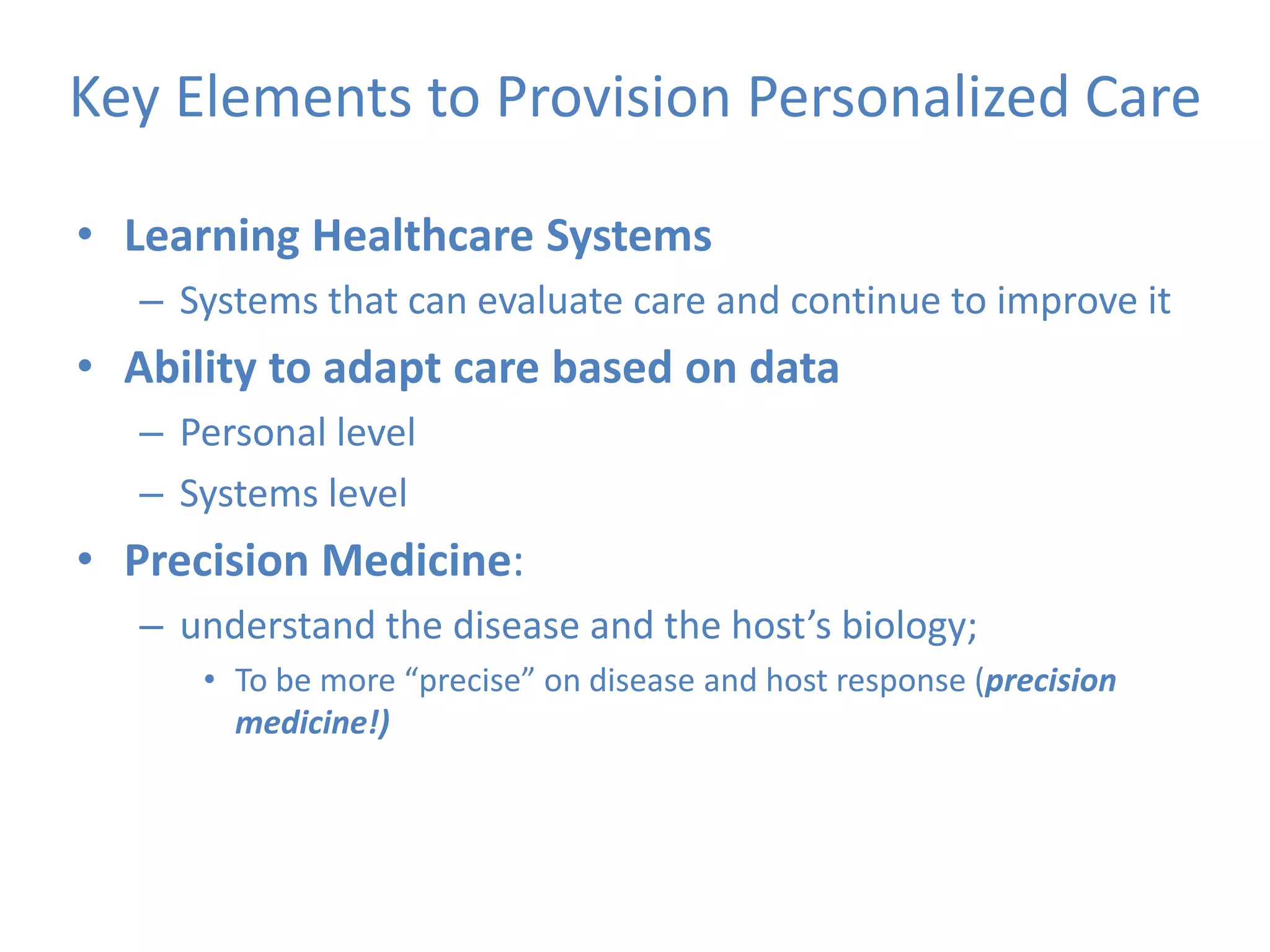
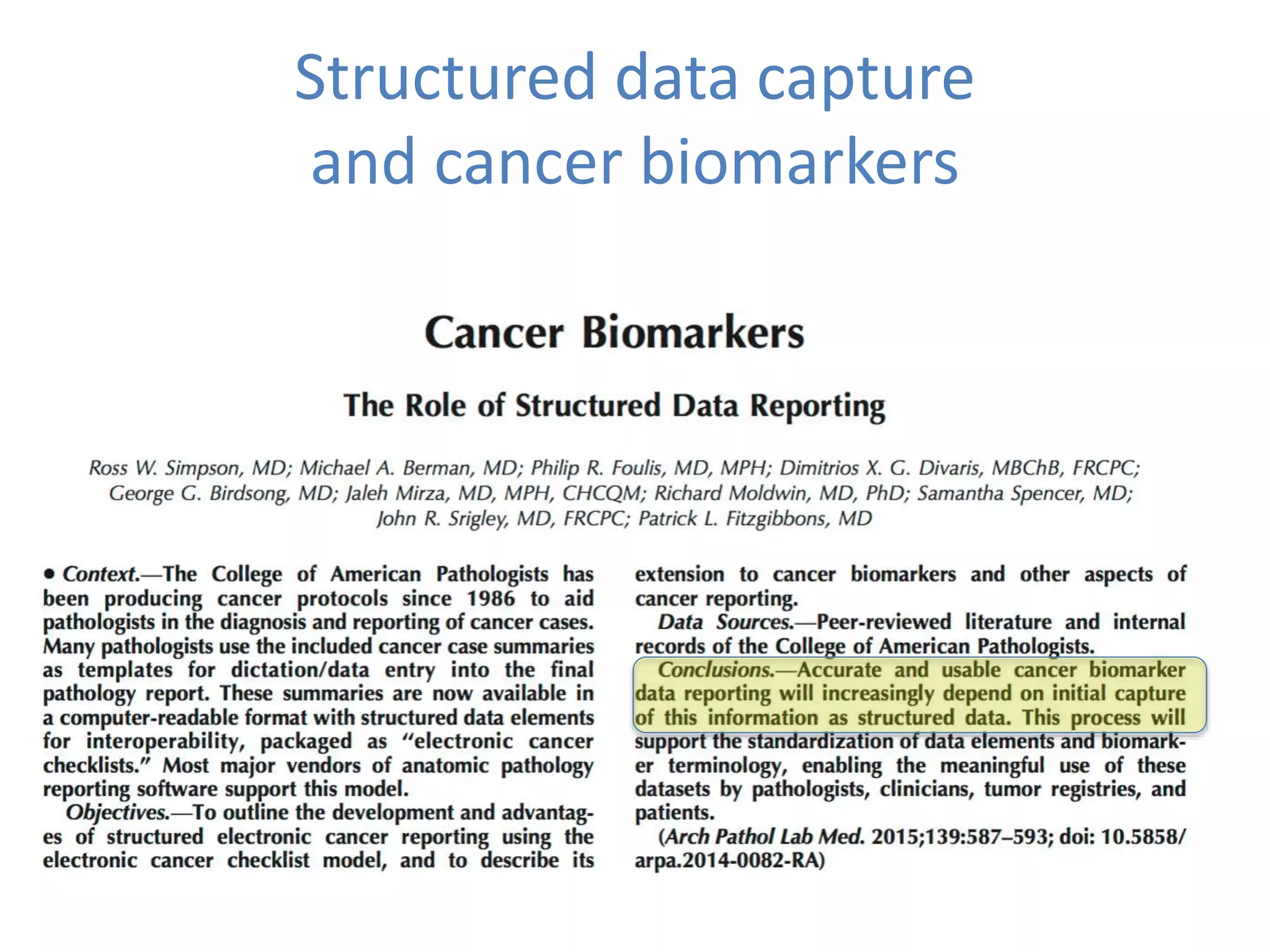
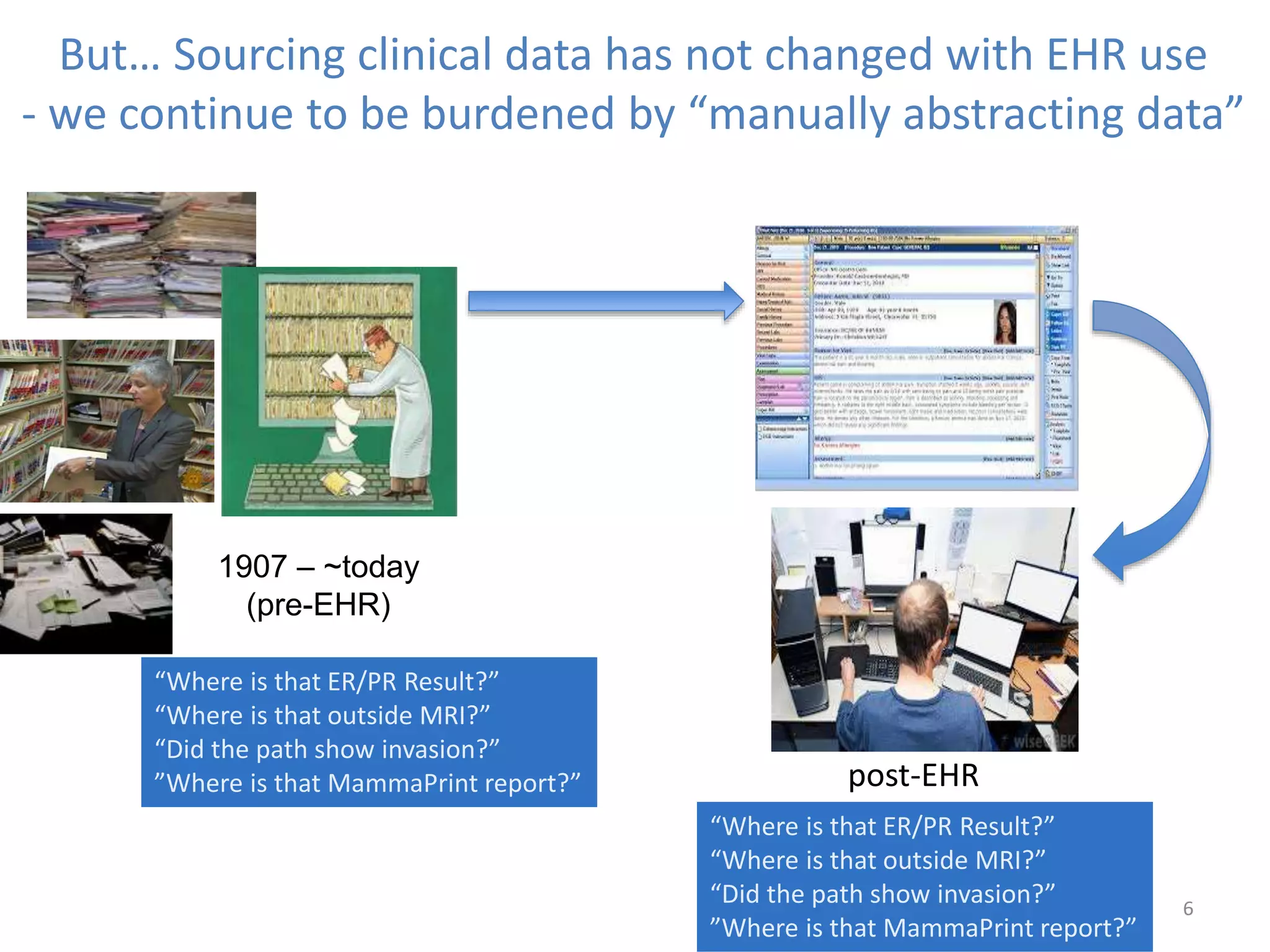
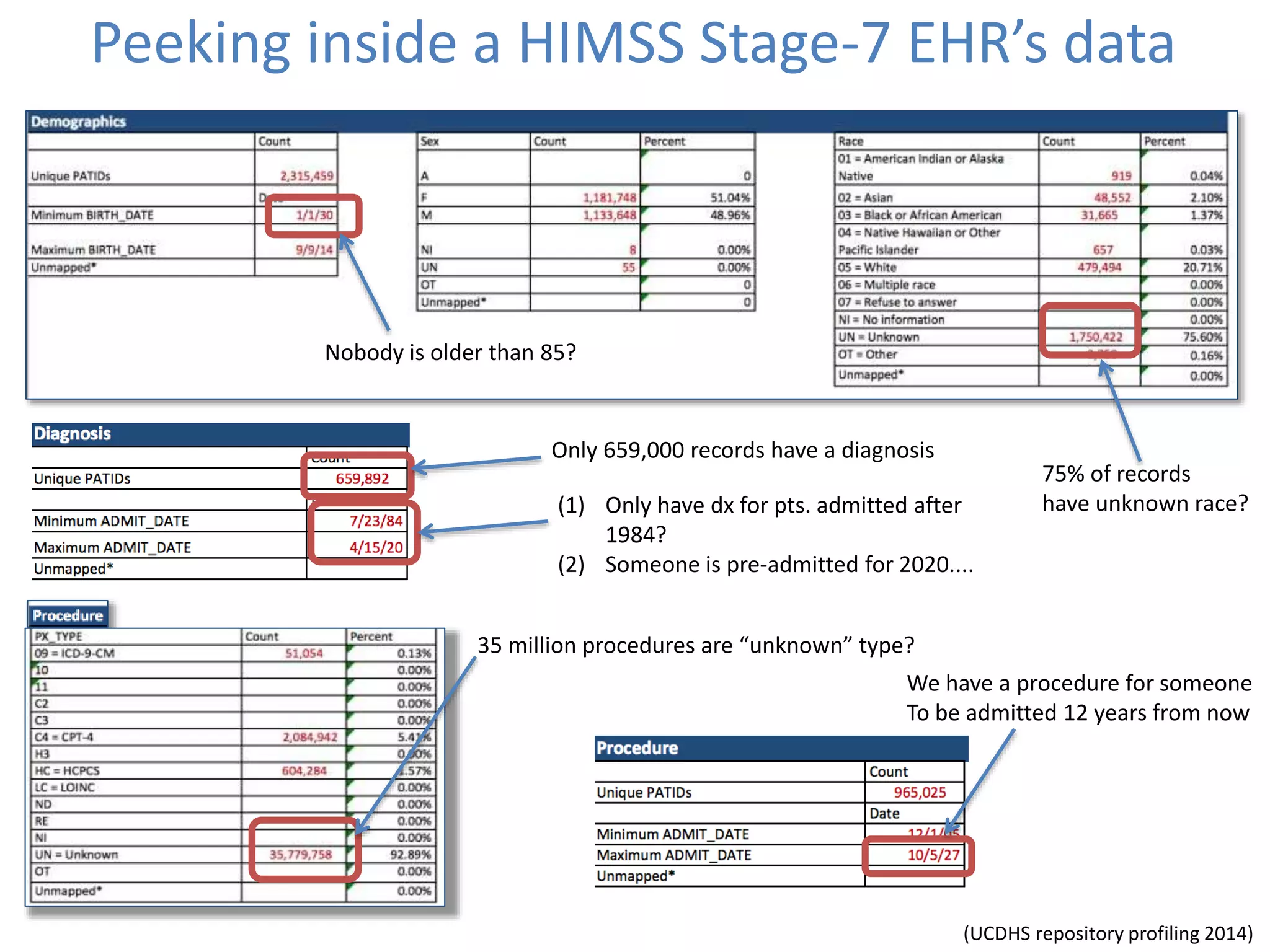
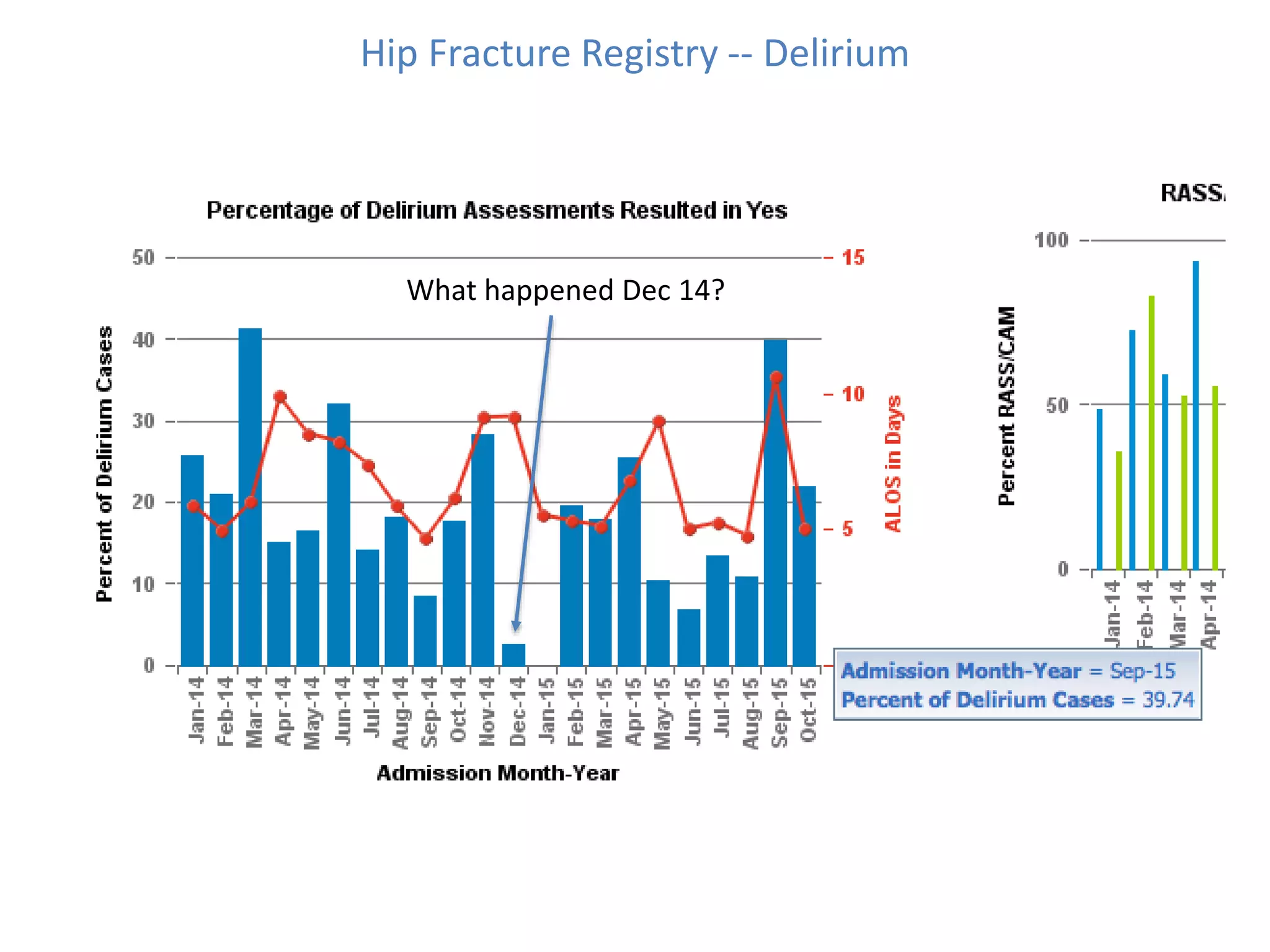
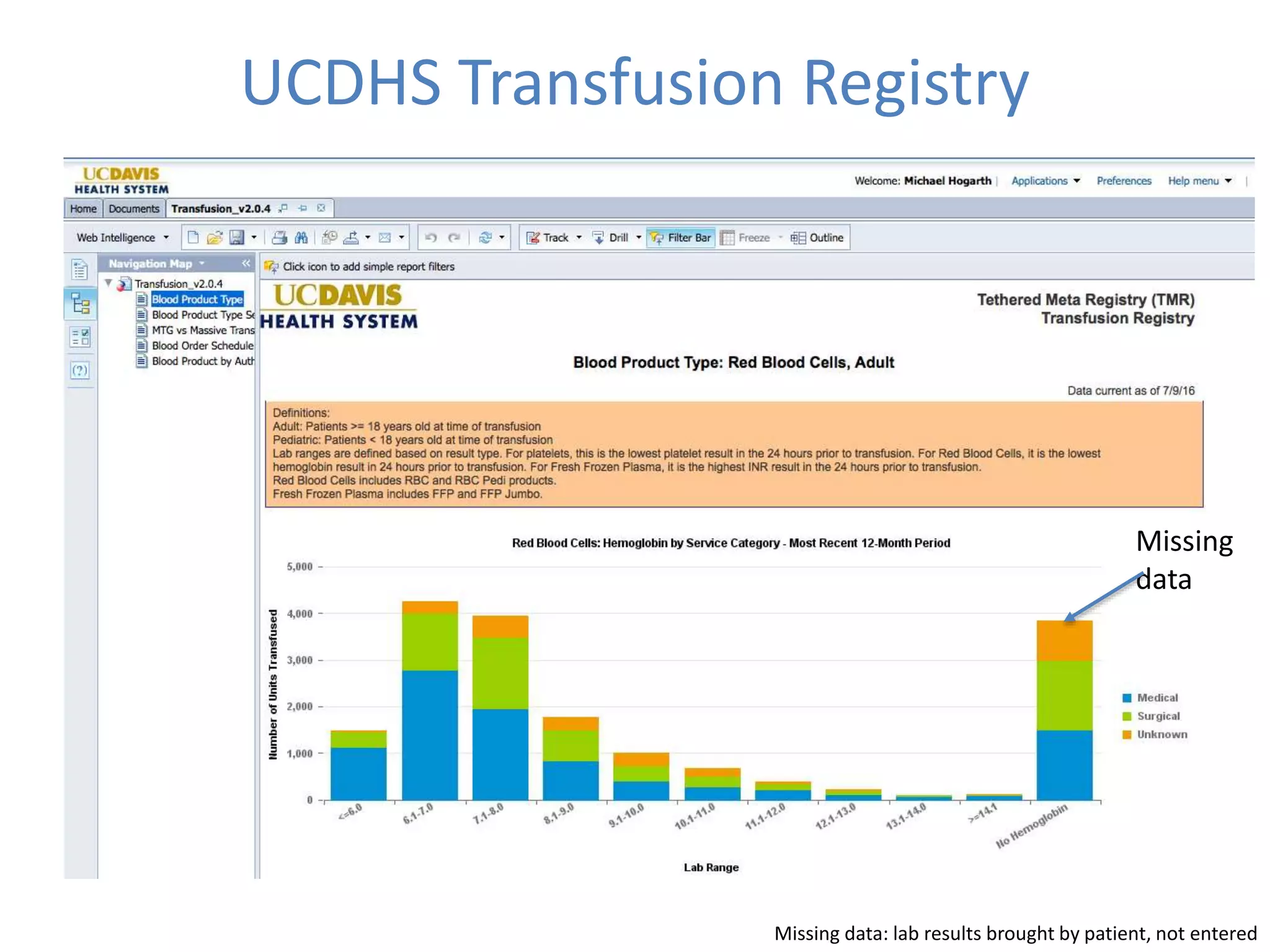
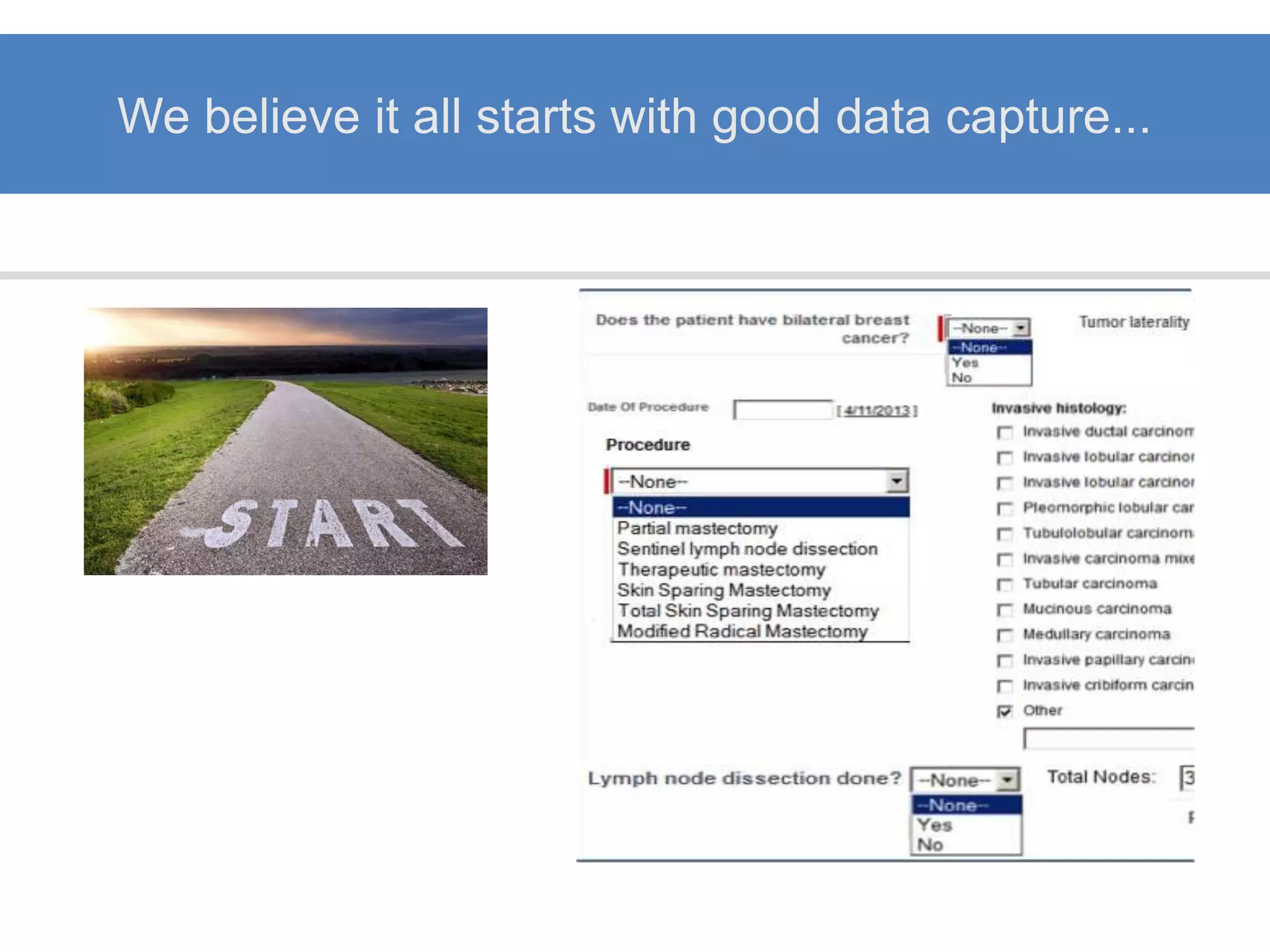
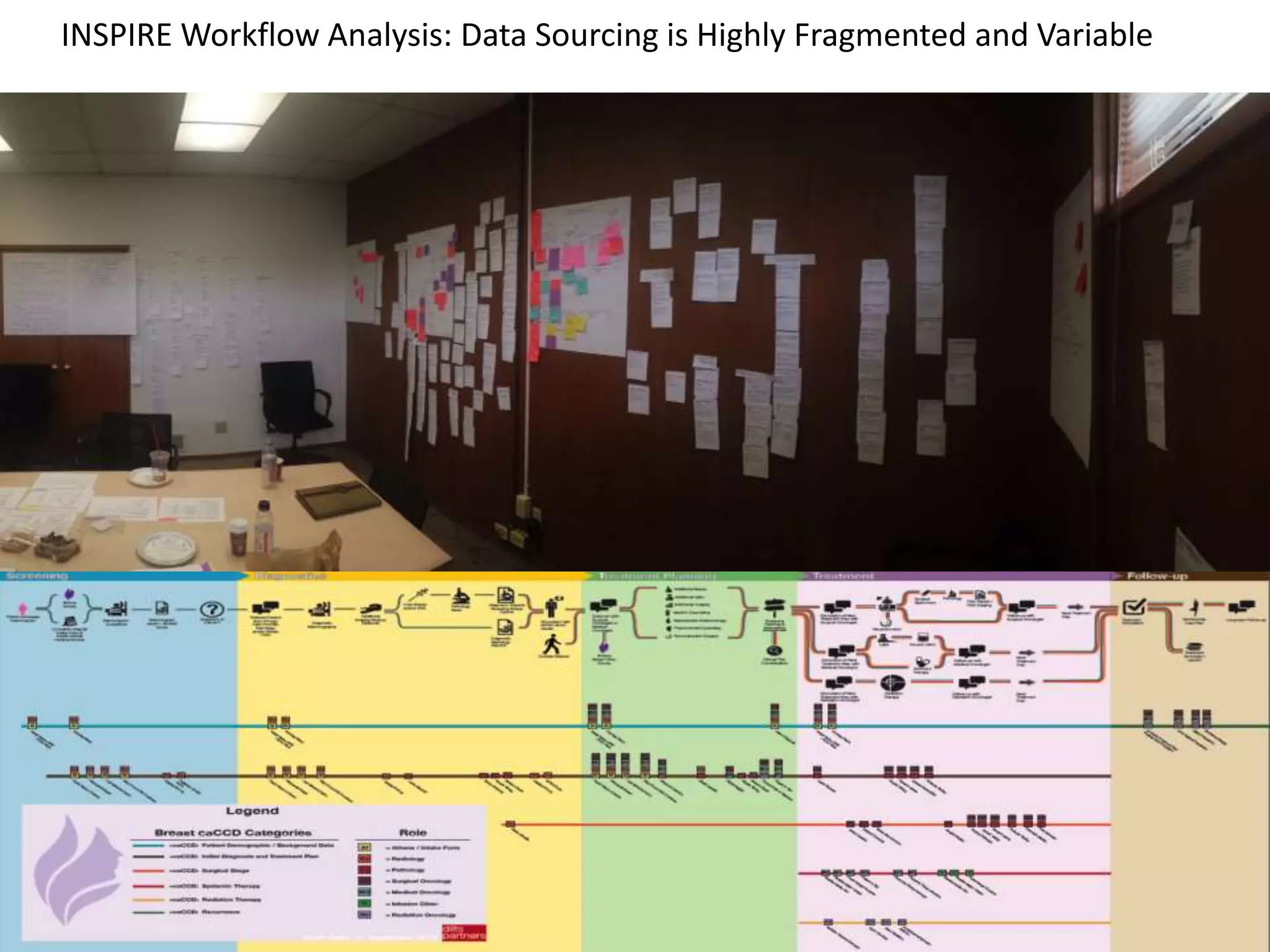
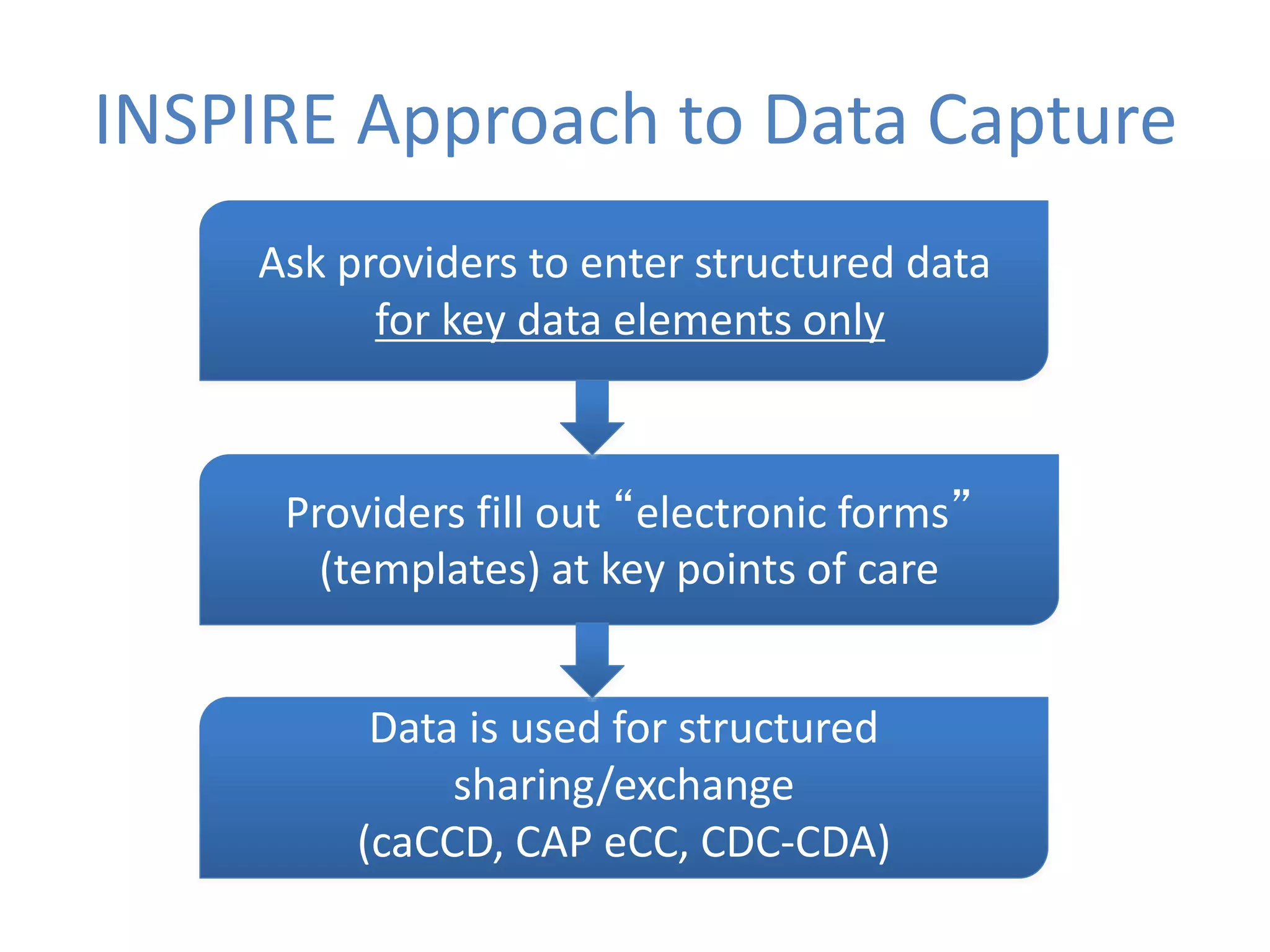
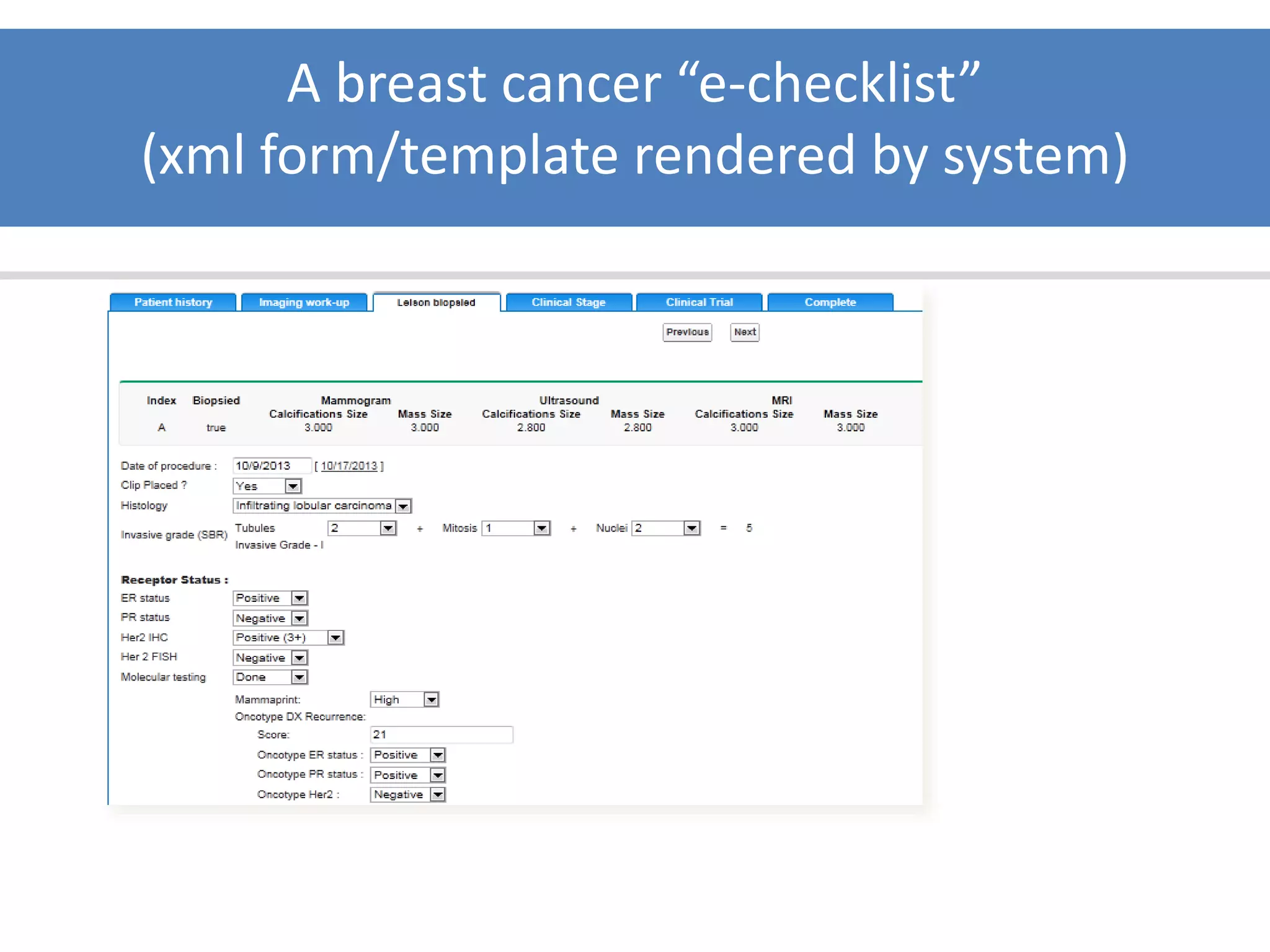
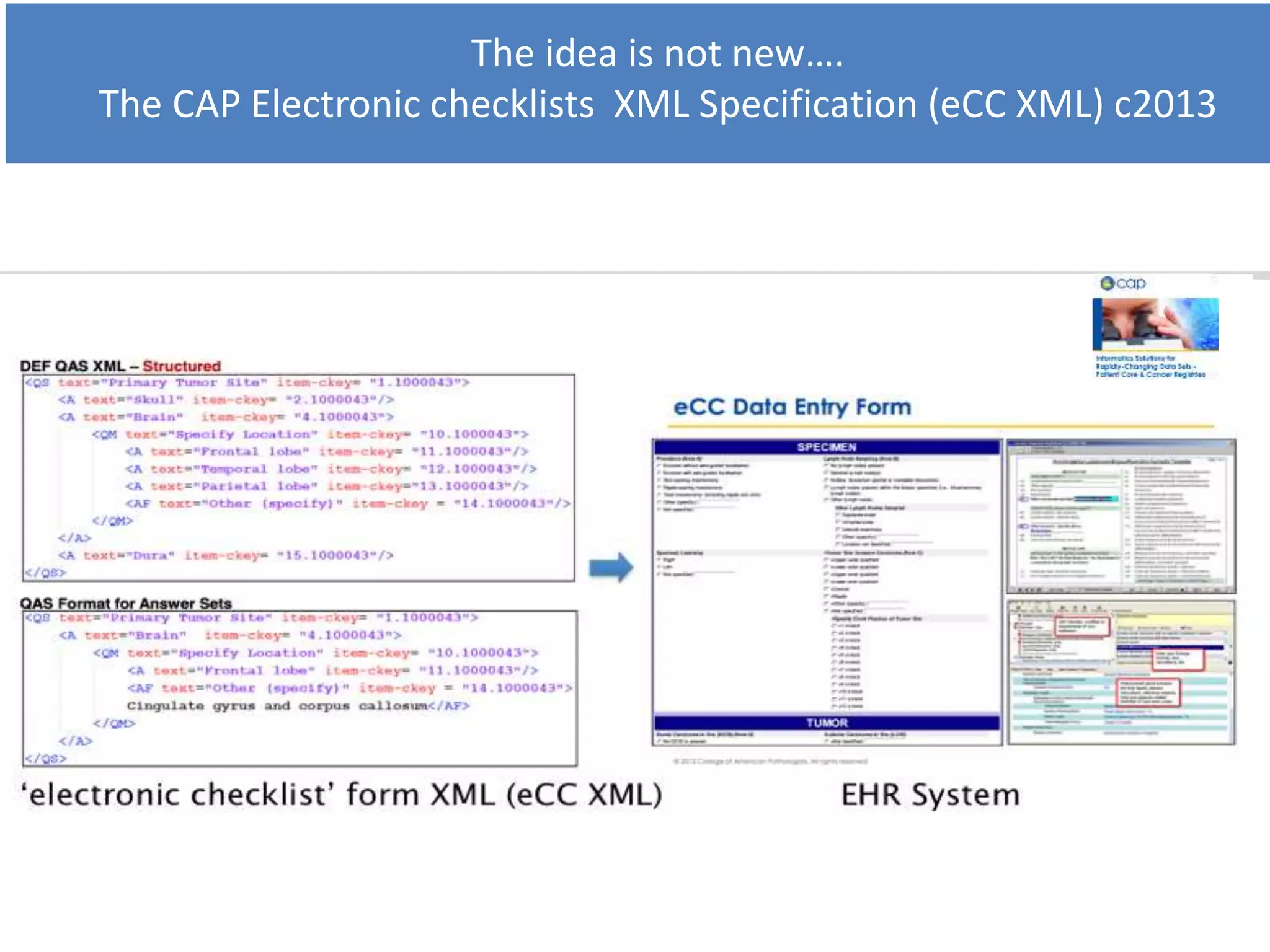
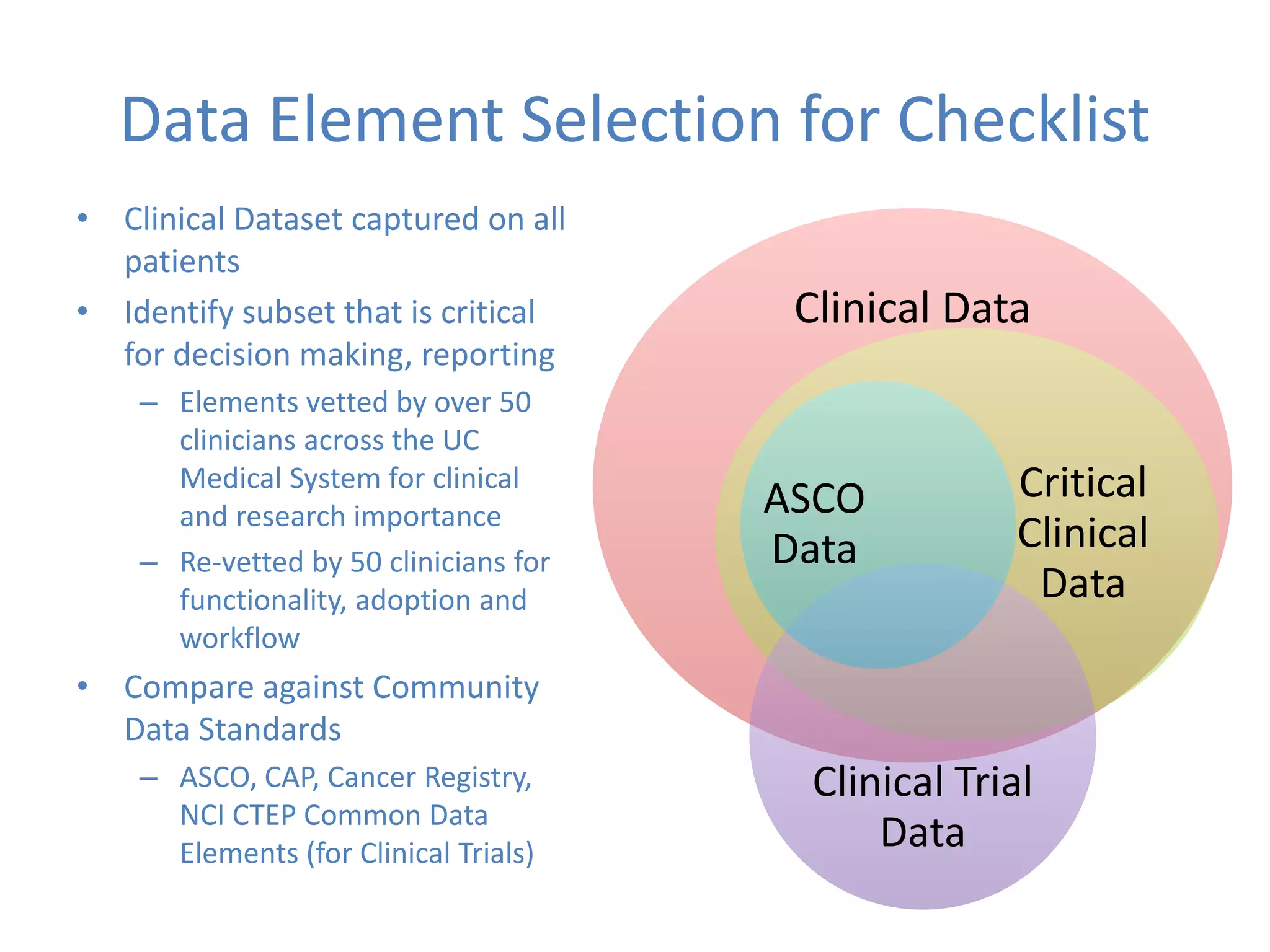
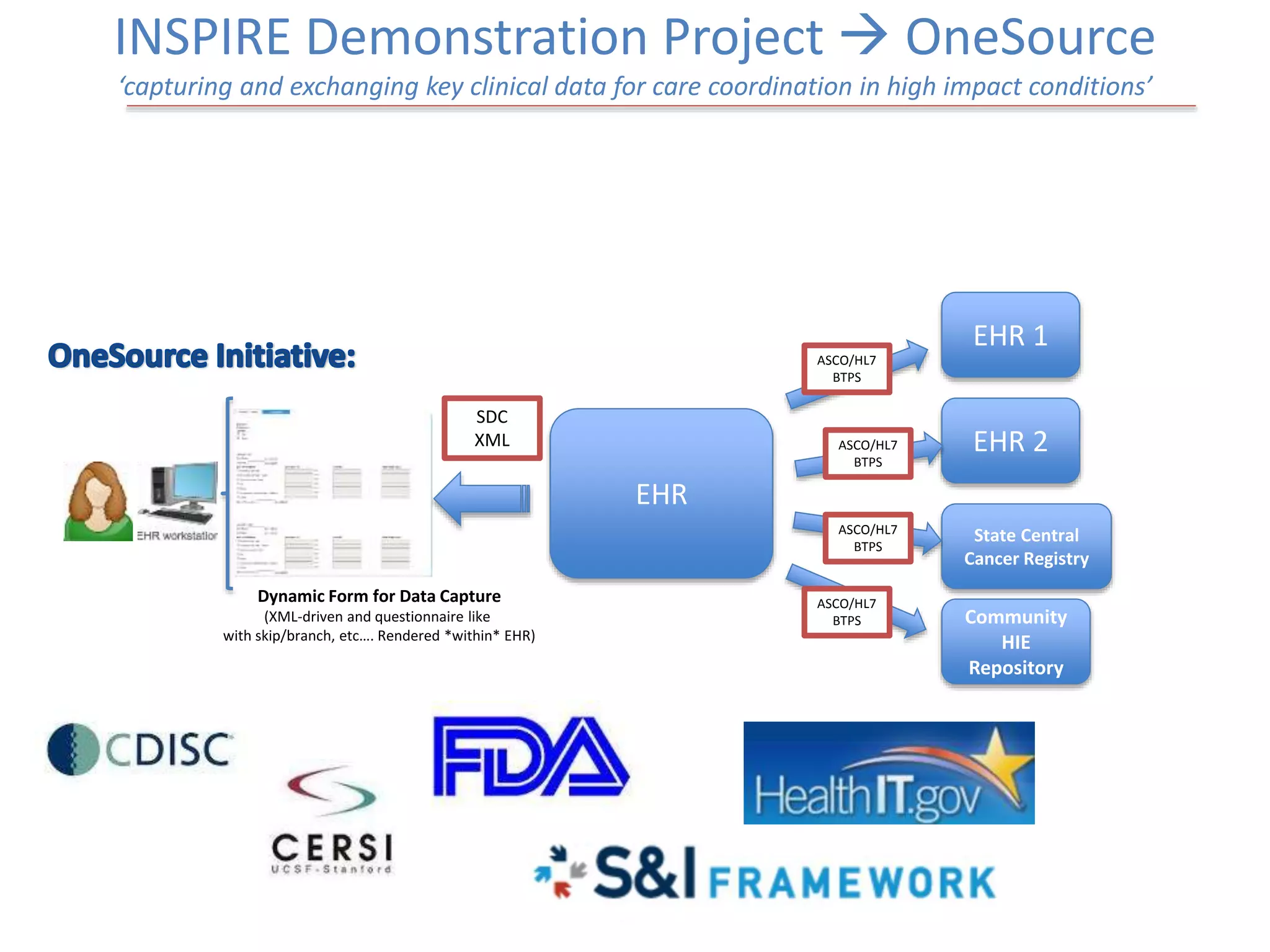
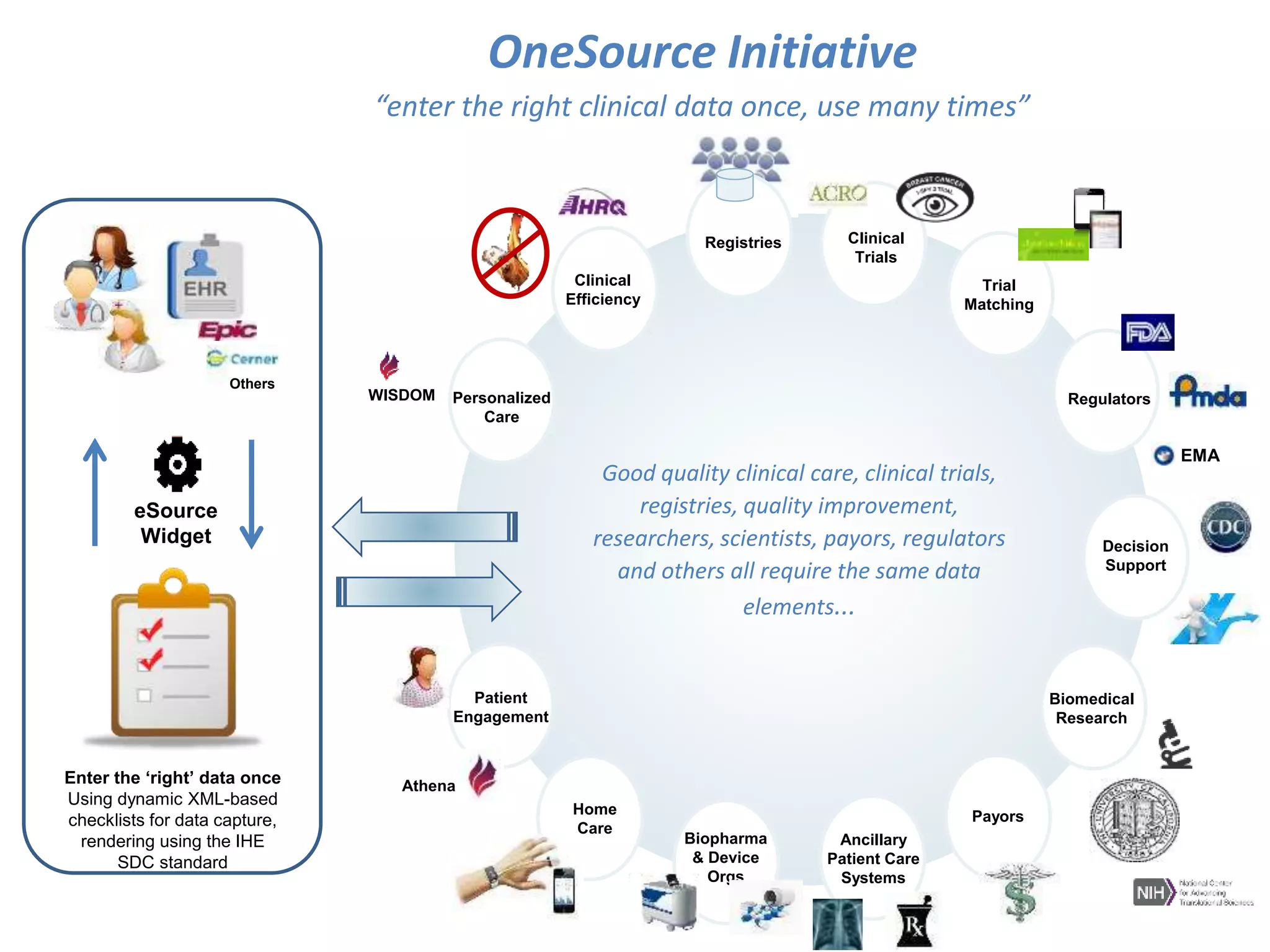
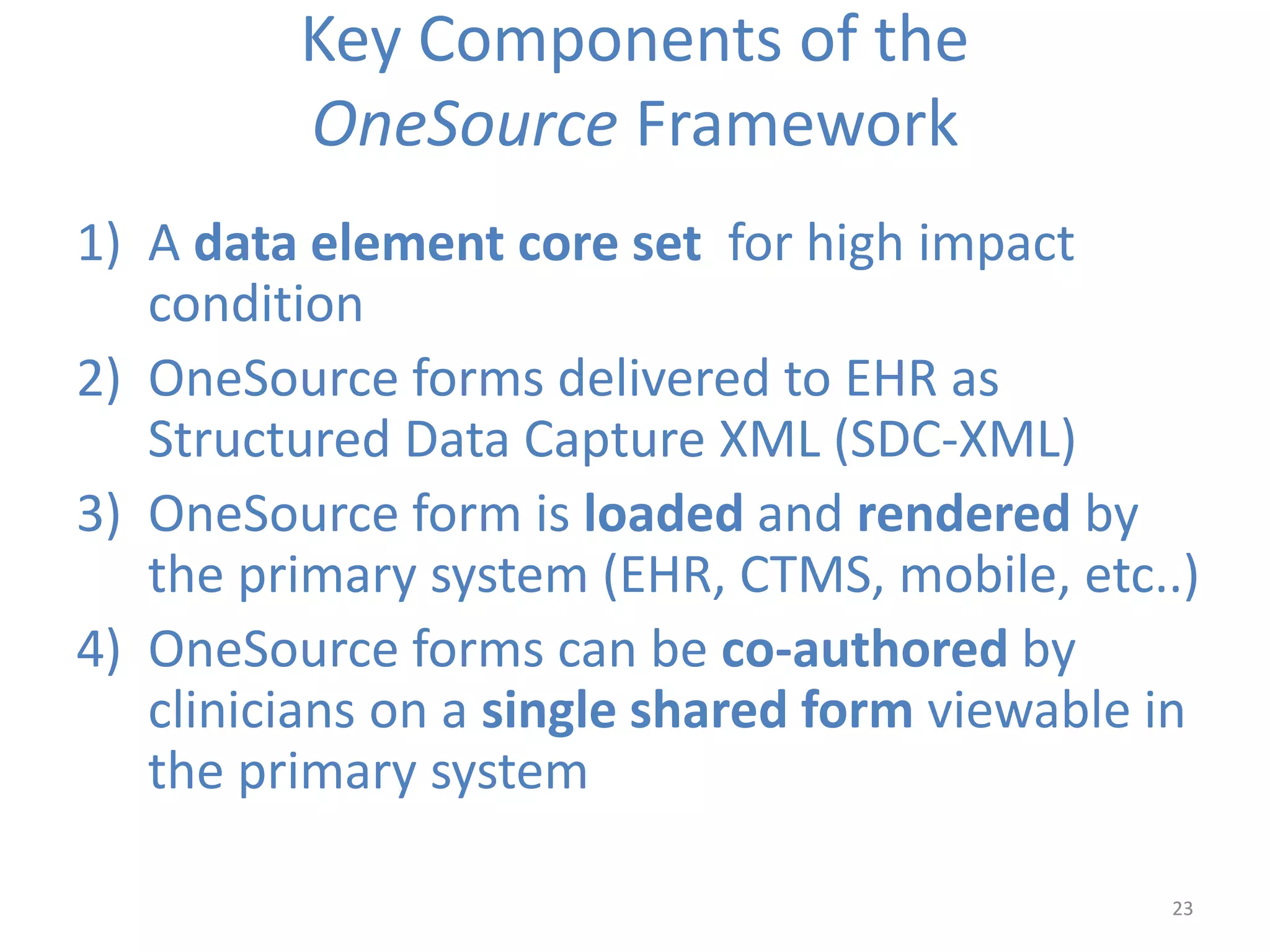
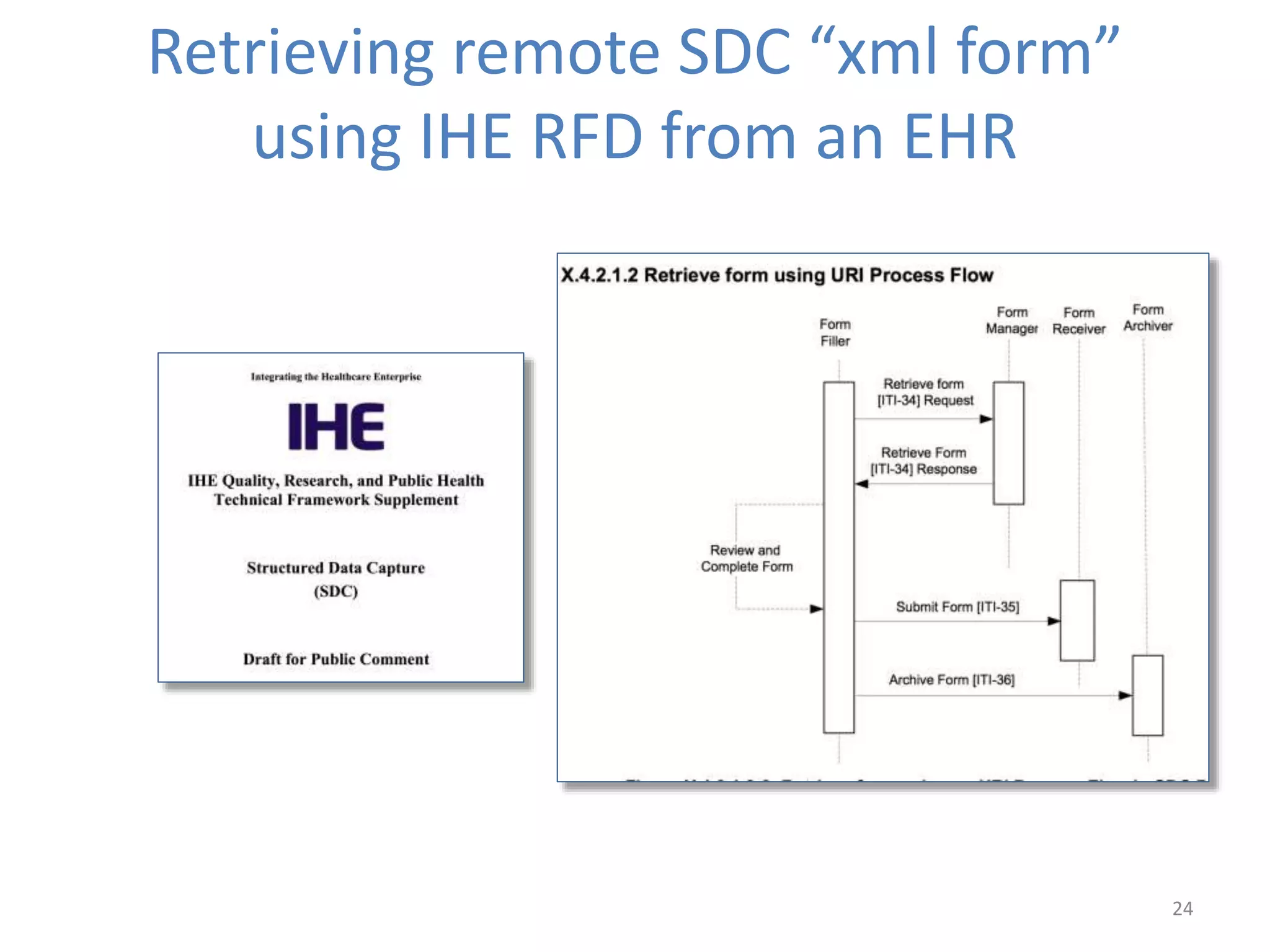
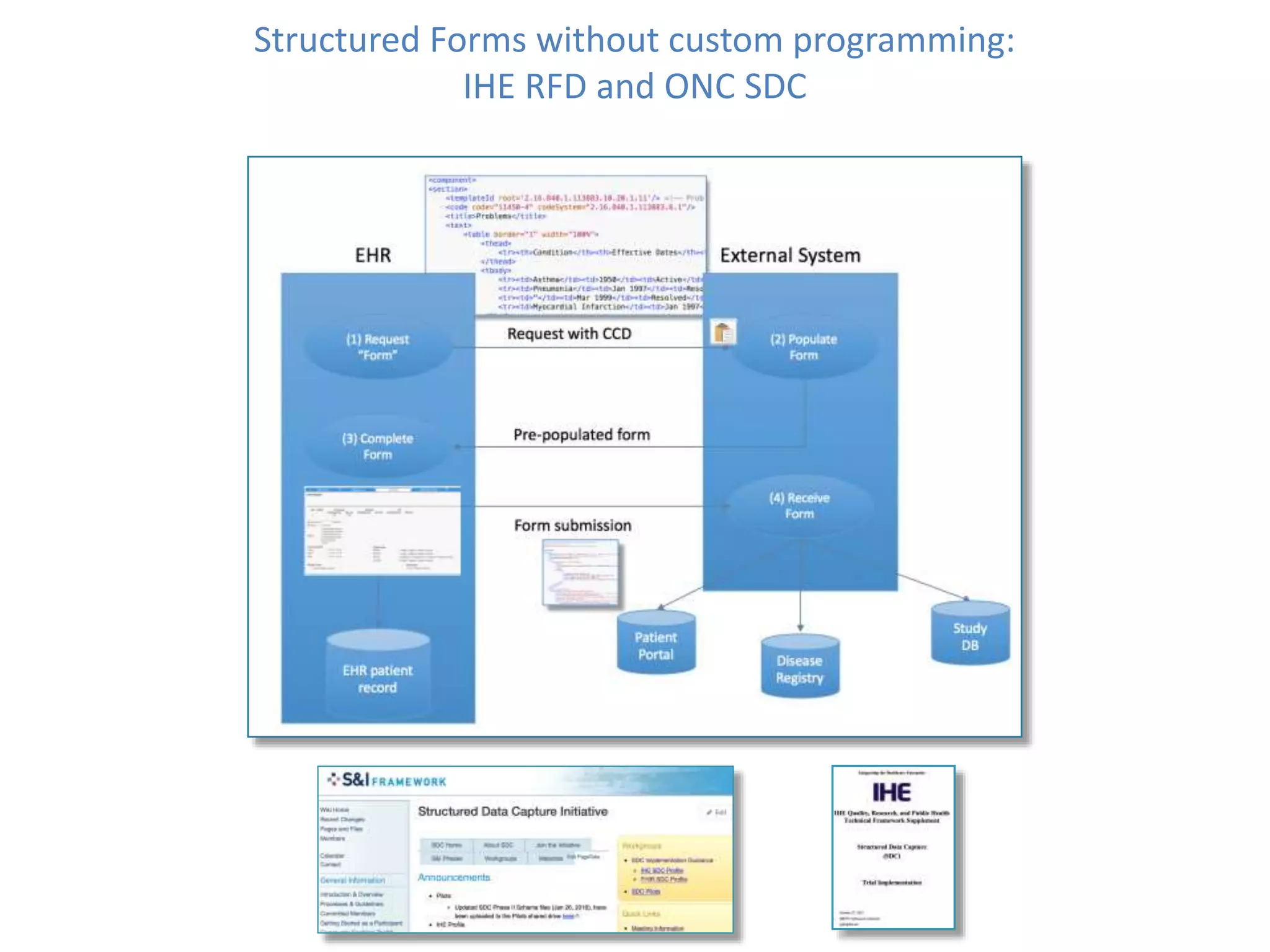
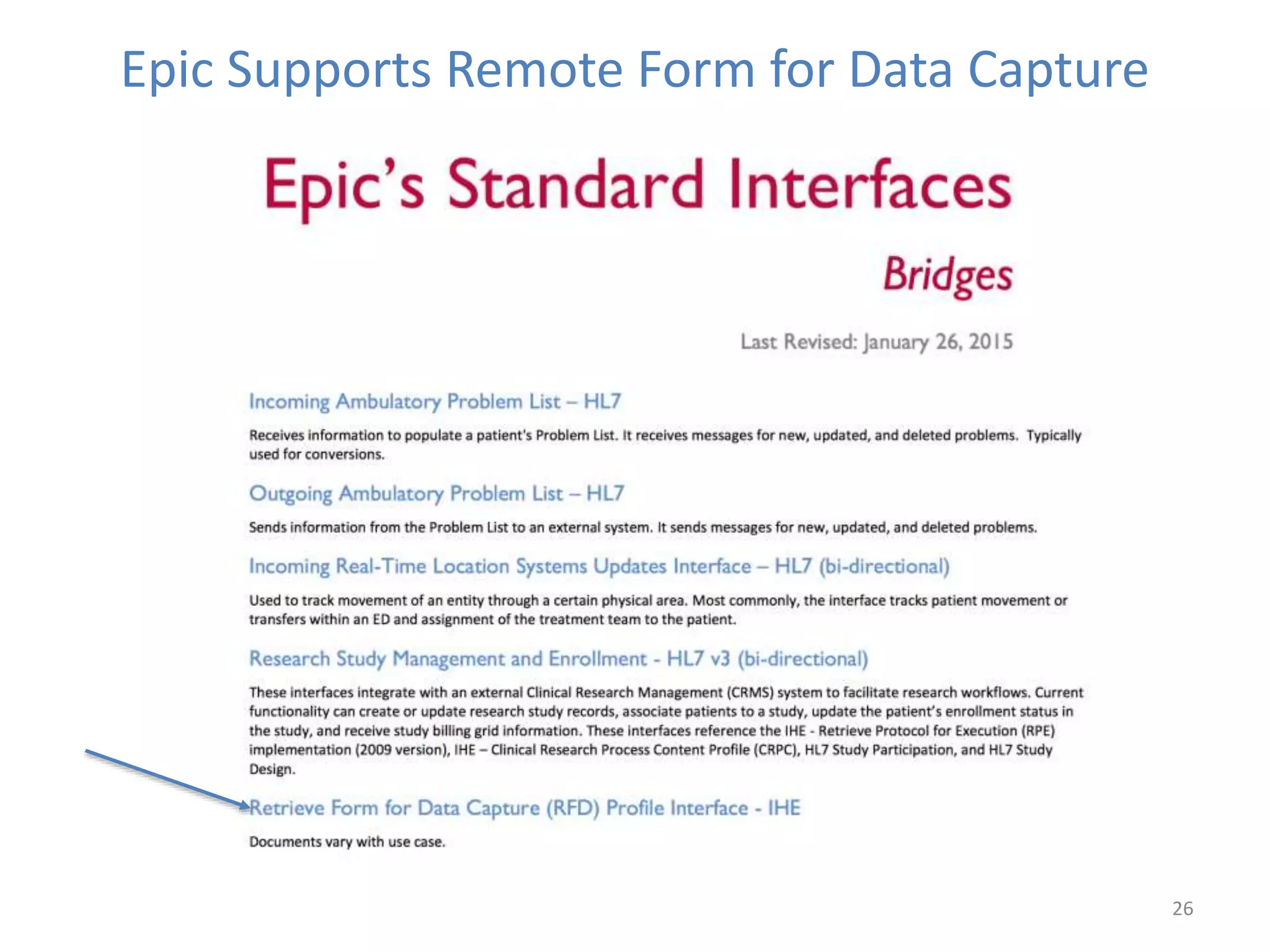
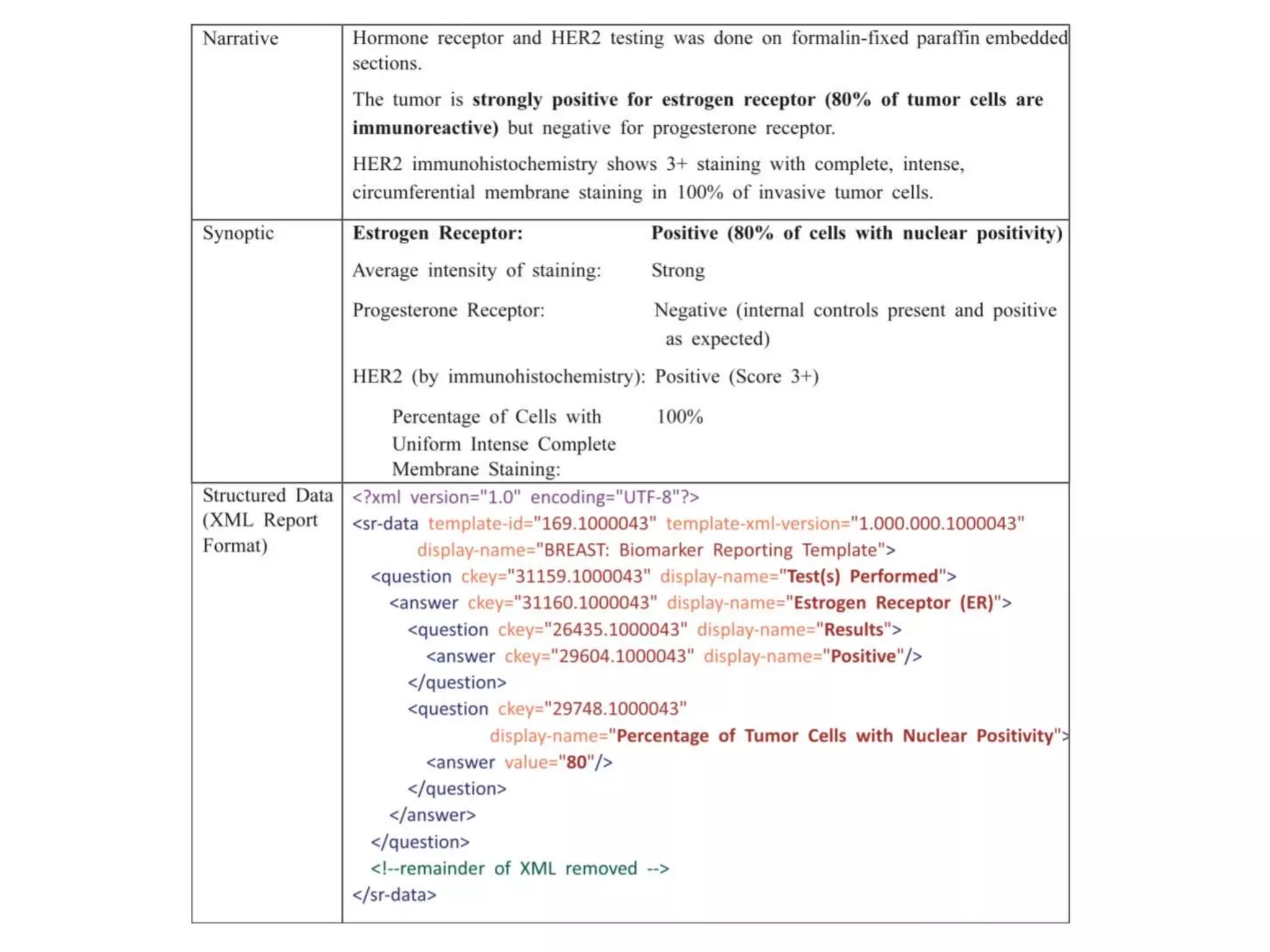
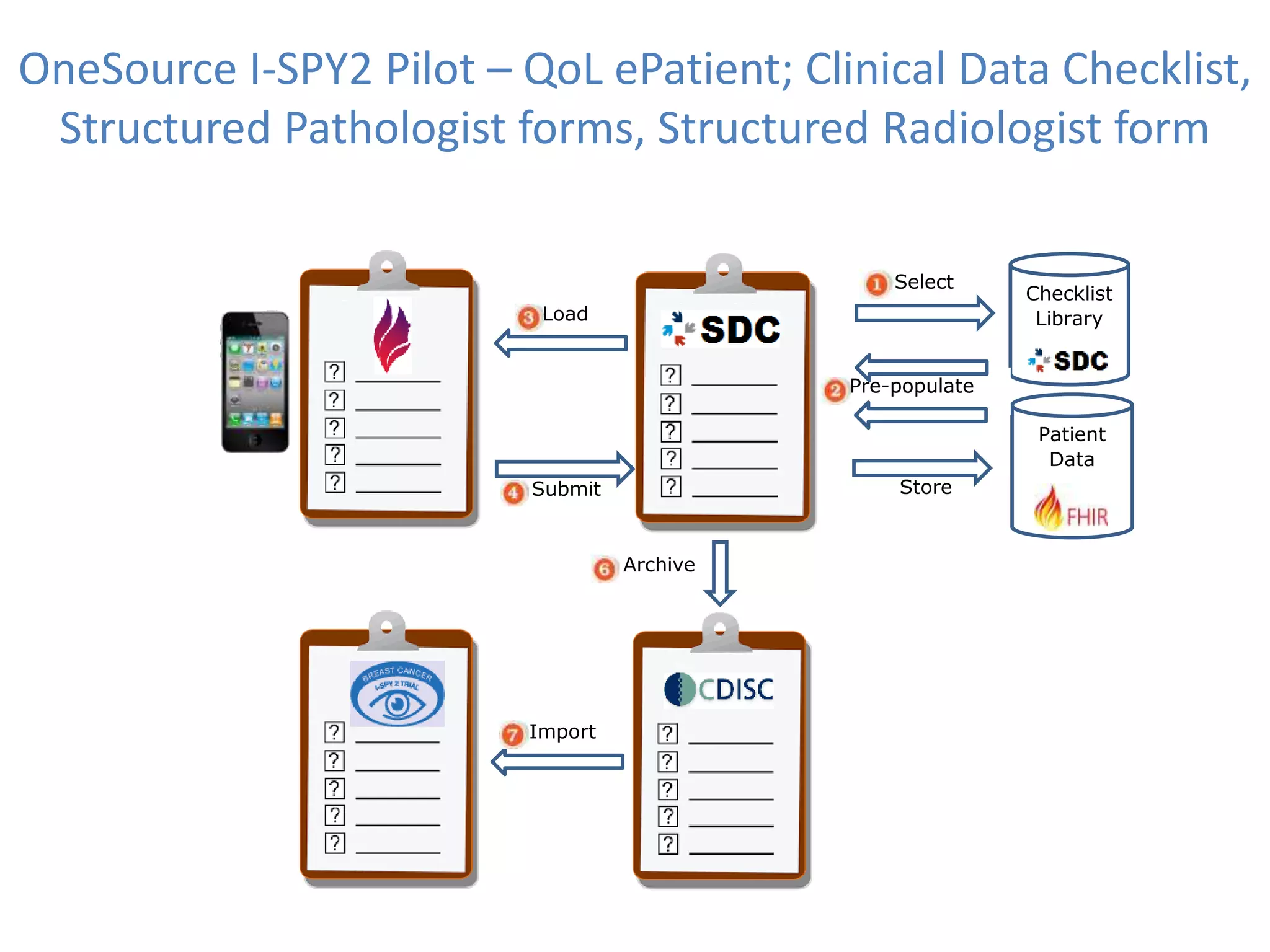
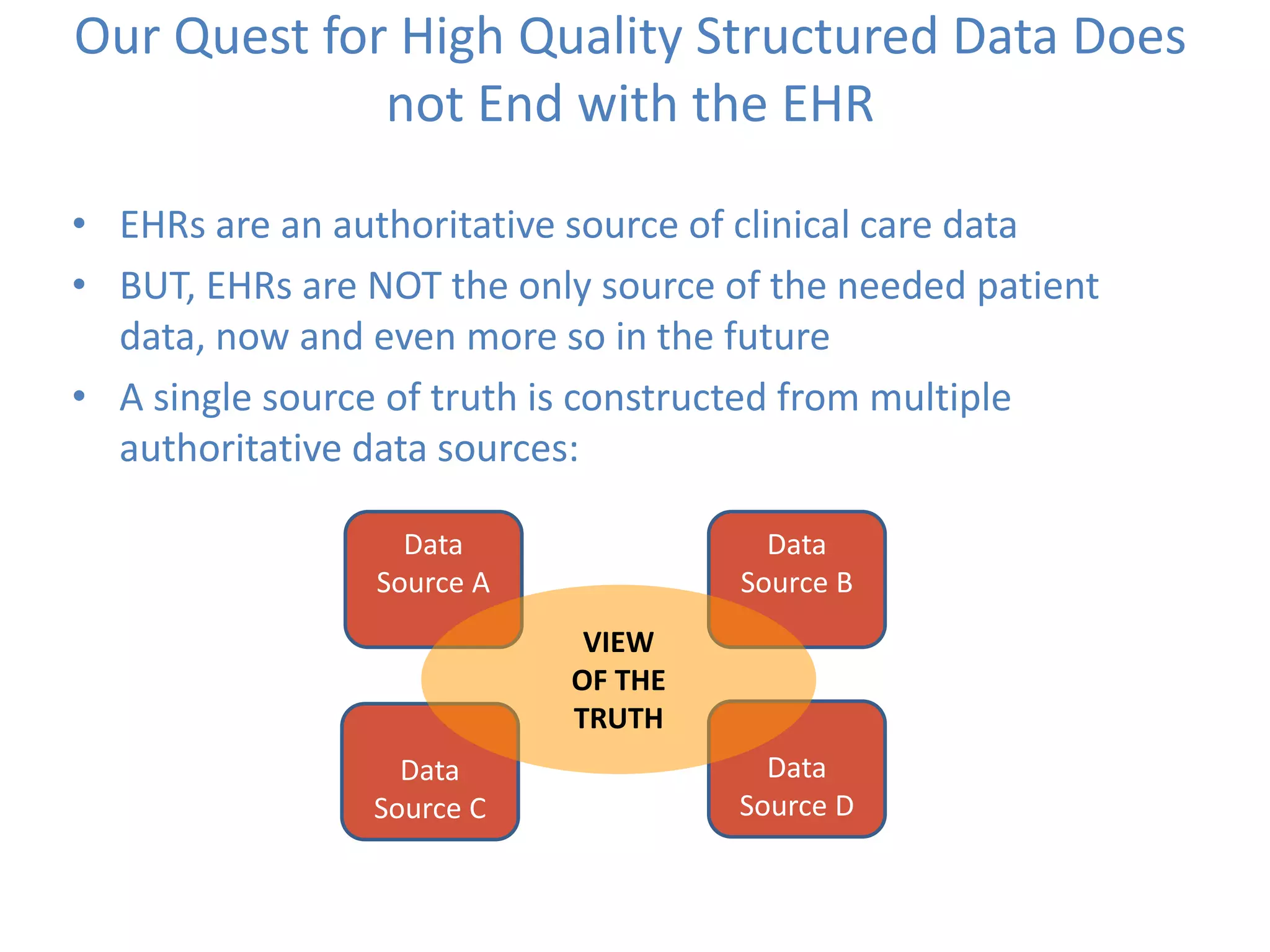
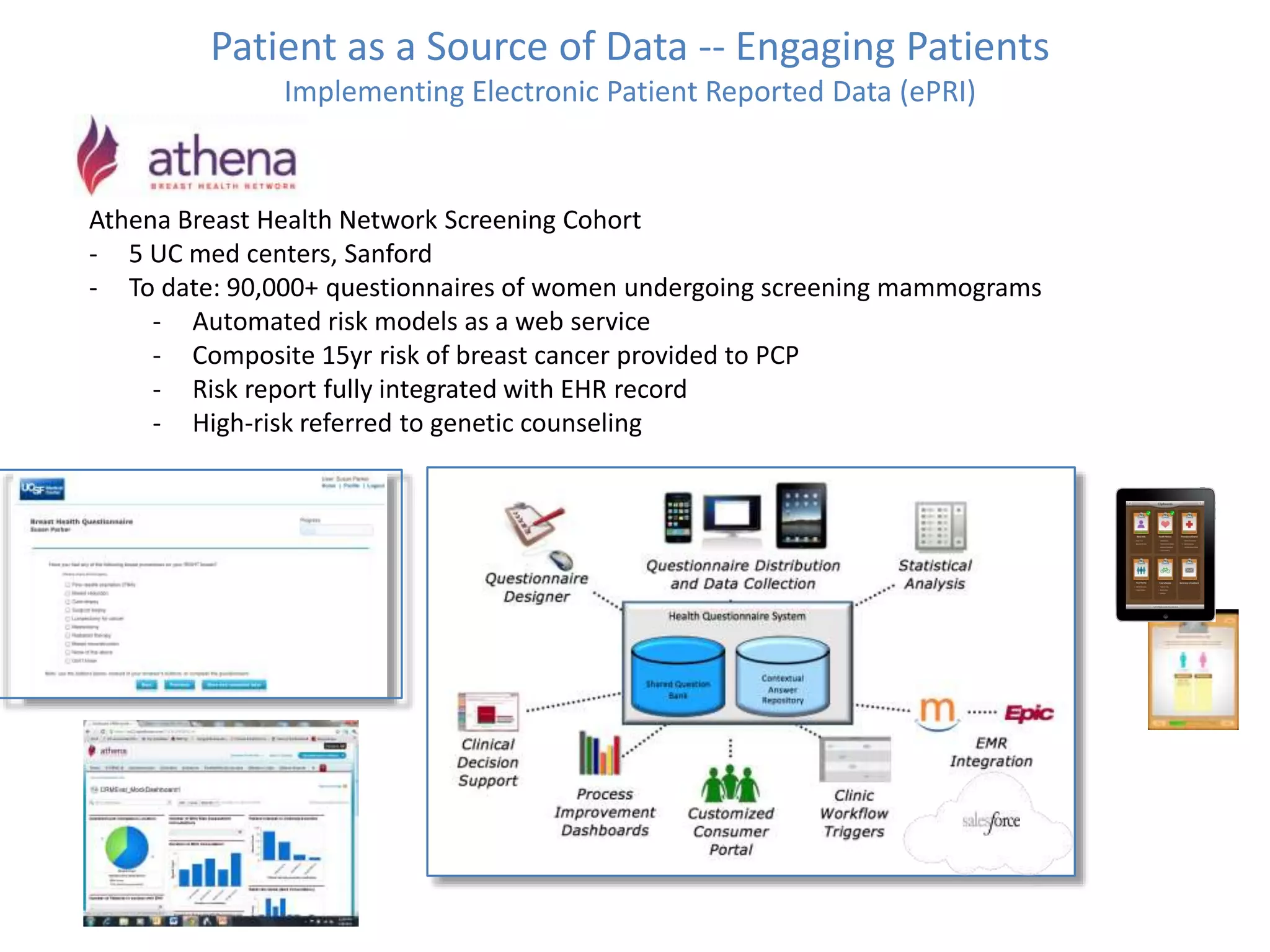
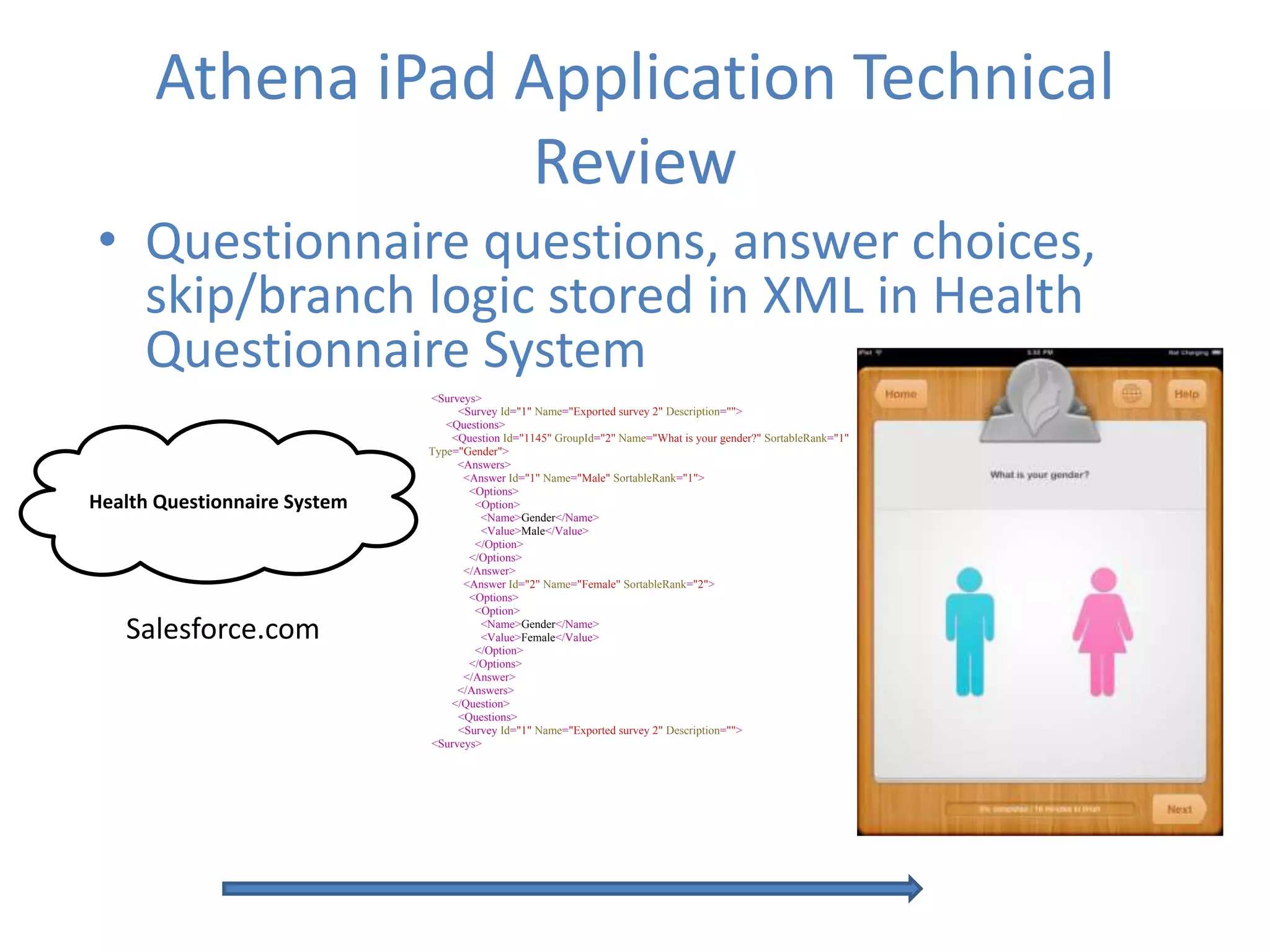
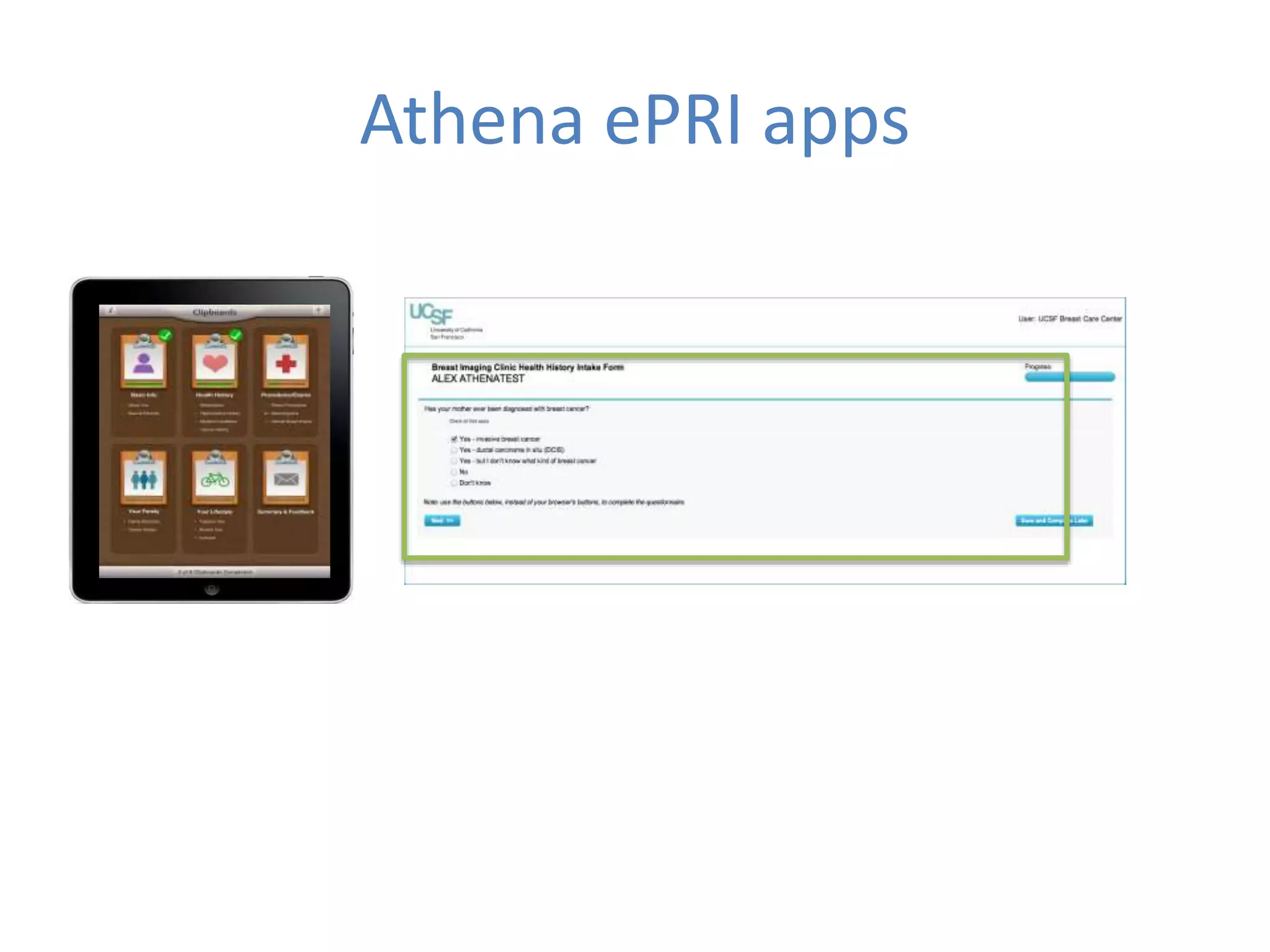
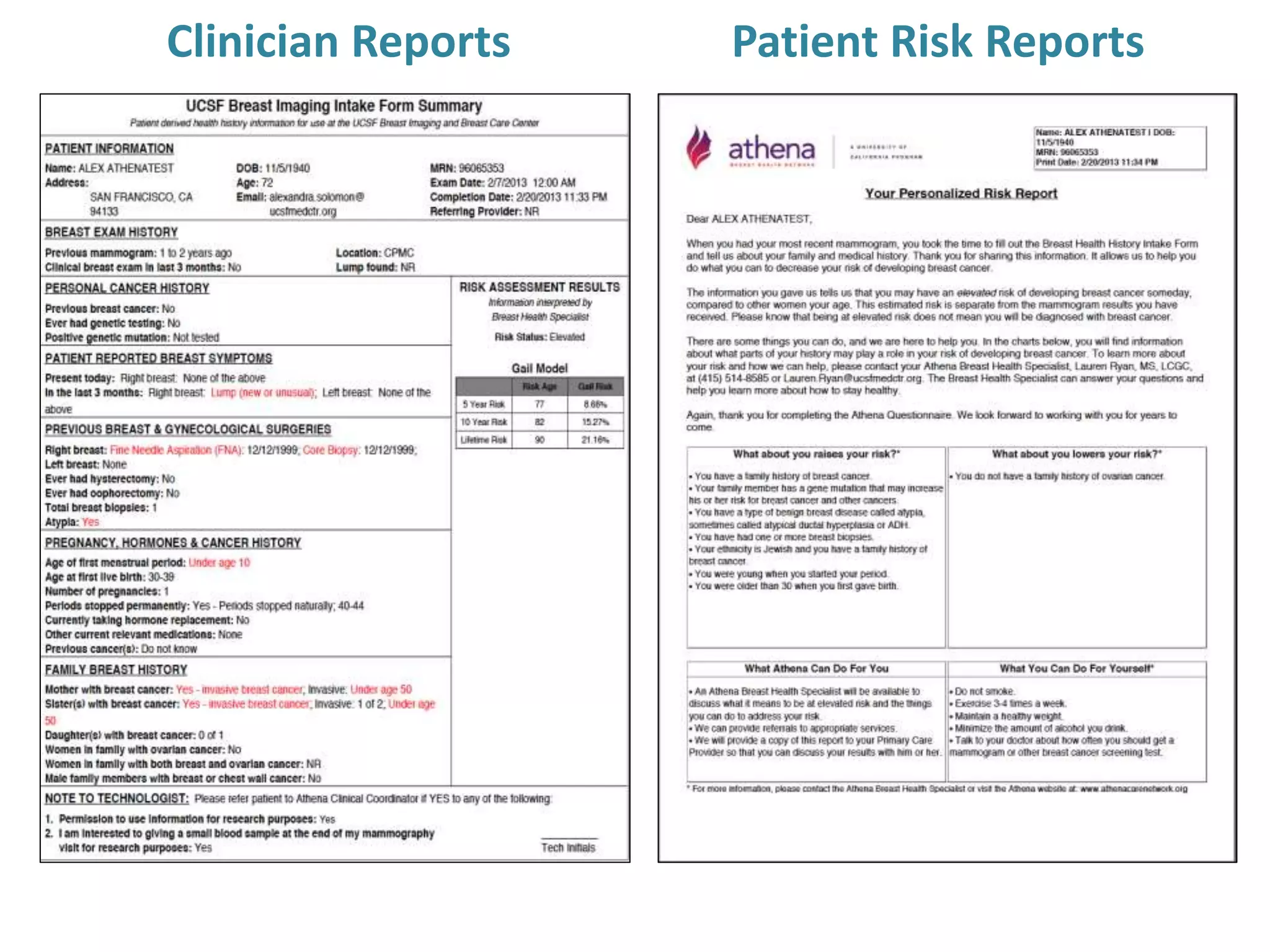
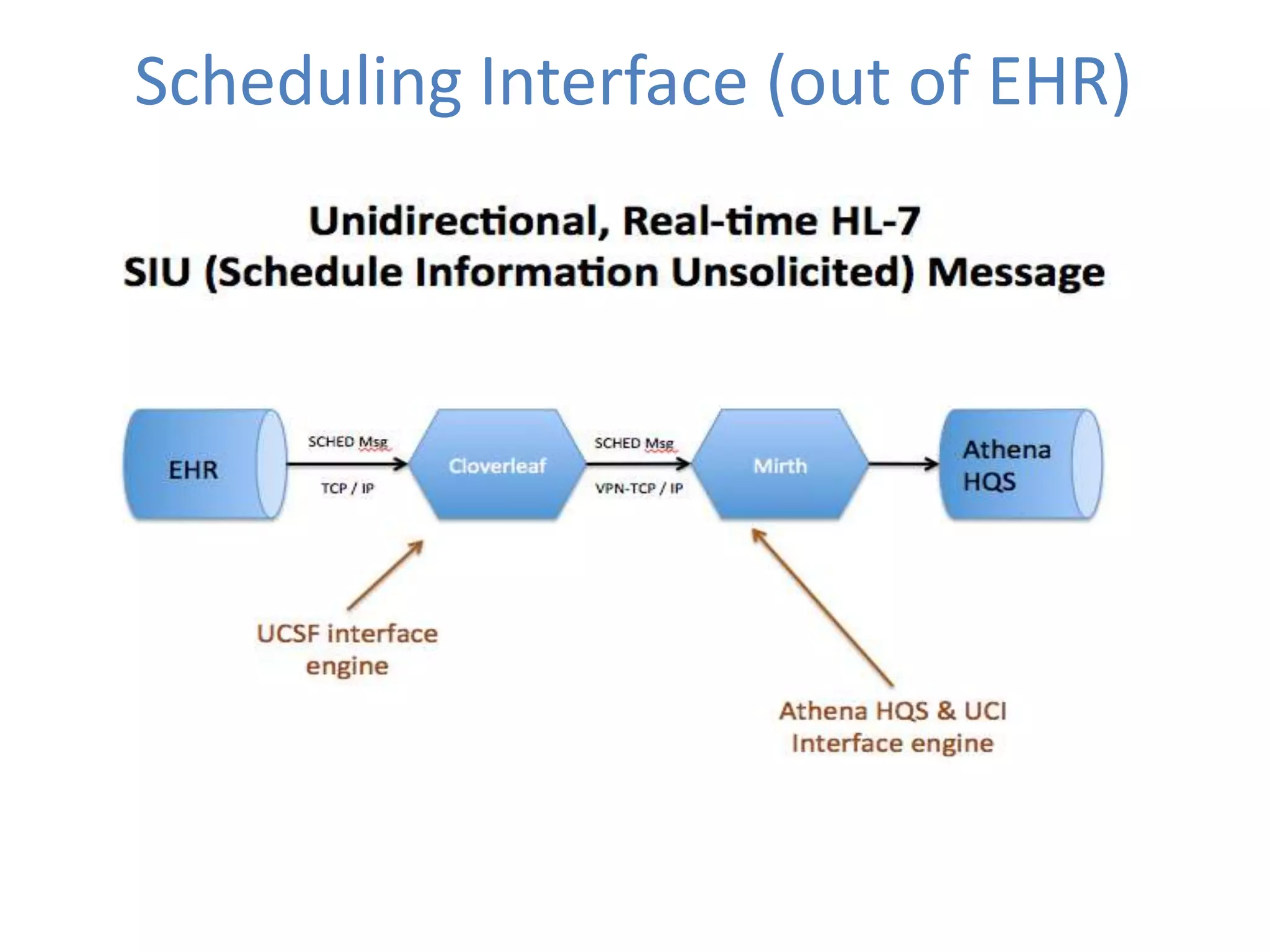
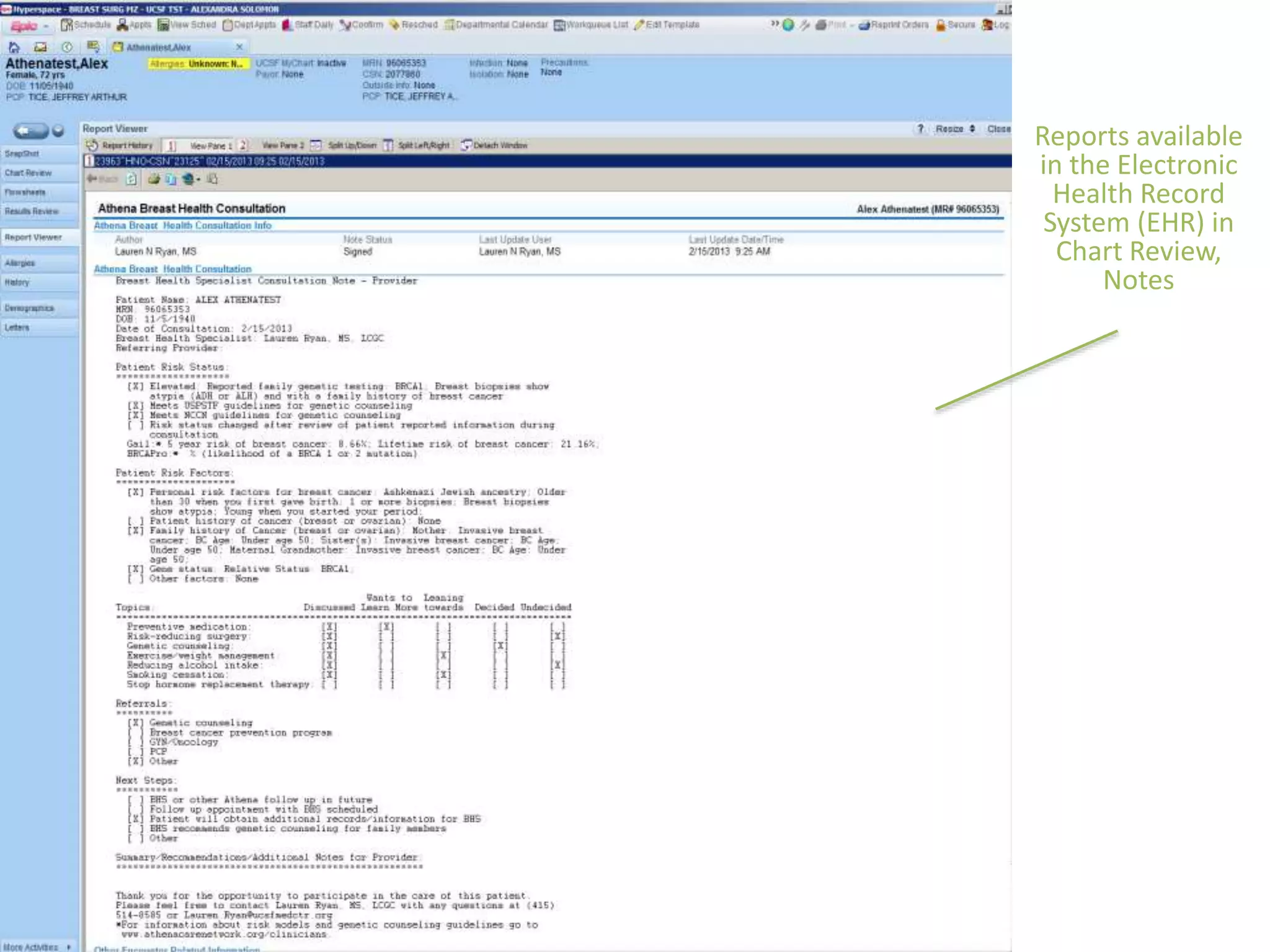
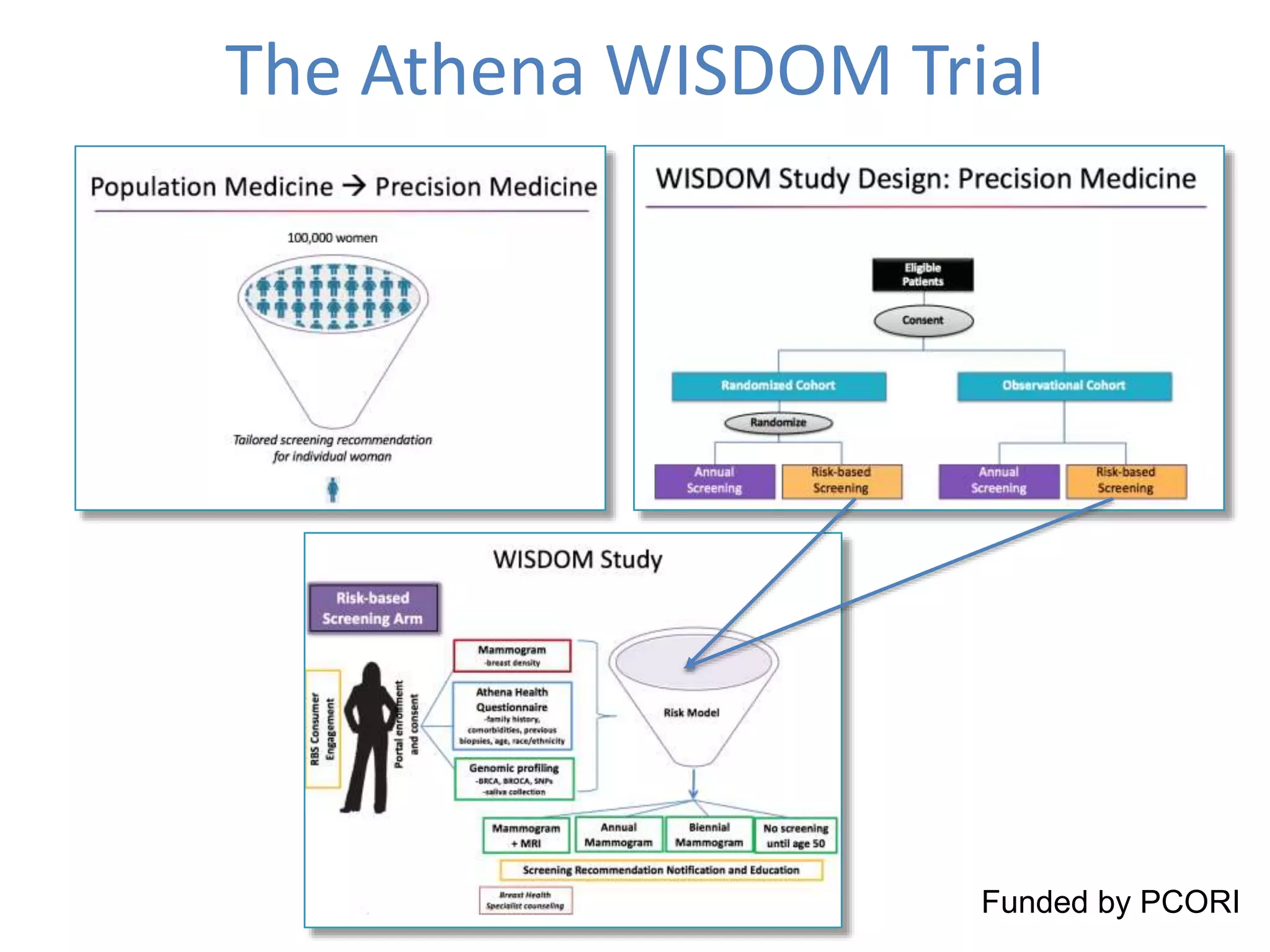
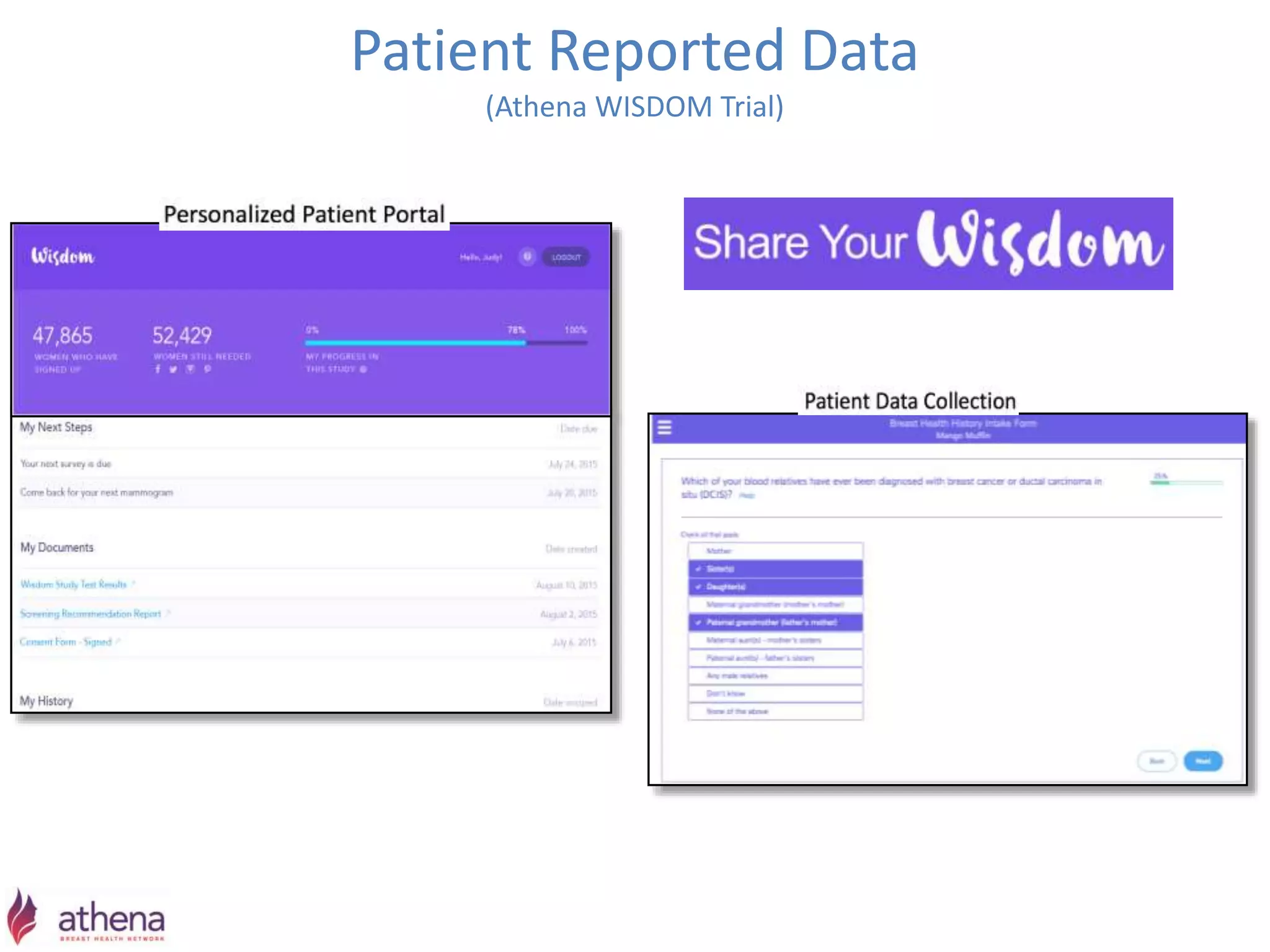
The Onesource Initiative aims to improve structured sourcing of key clinical data for personalized care, addressing challenges in data capture and documentation within electronic health records (EHRs). It advocates for a transformation in clinical documentation through the use of dynamic XML-based checklists to enhance data quality, streamline information retrieval, and support clinical decision-making. Inspired by trials demonstrating fragmented data workflows, the initiative emphasizes the importance of structured sharing for efficient care coordination and engaging patients in data reporting.