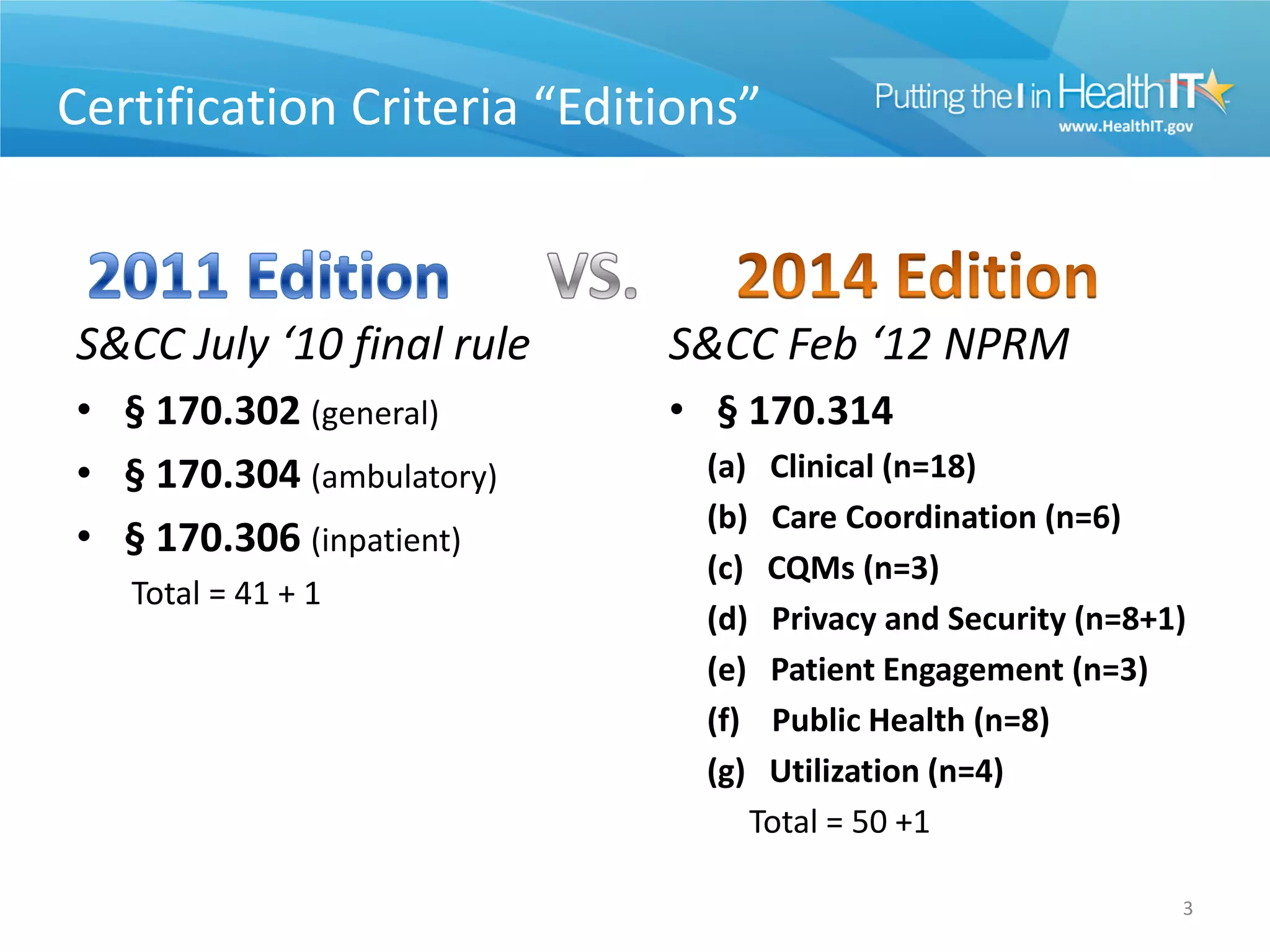
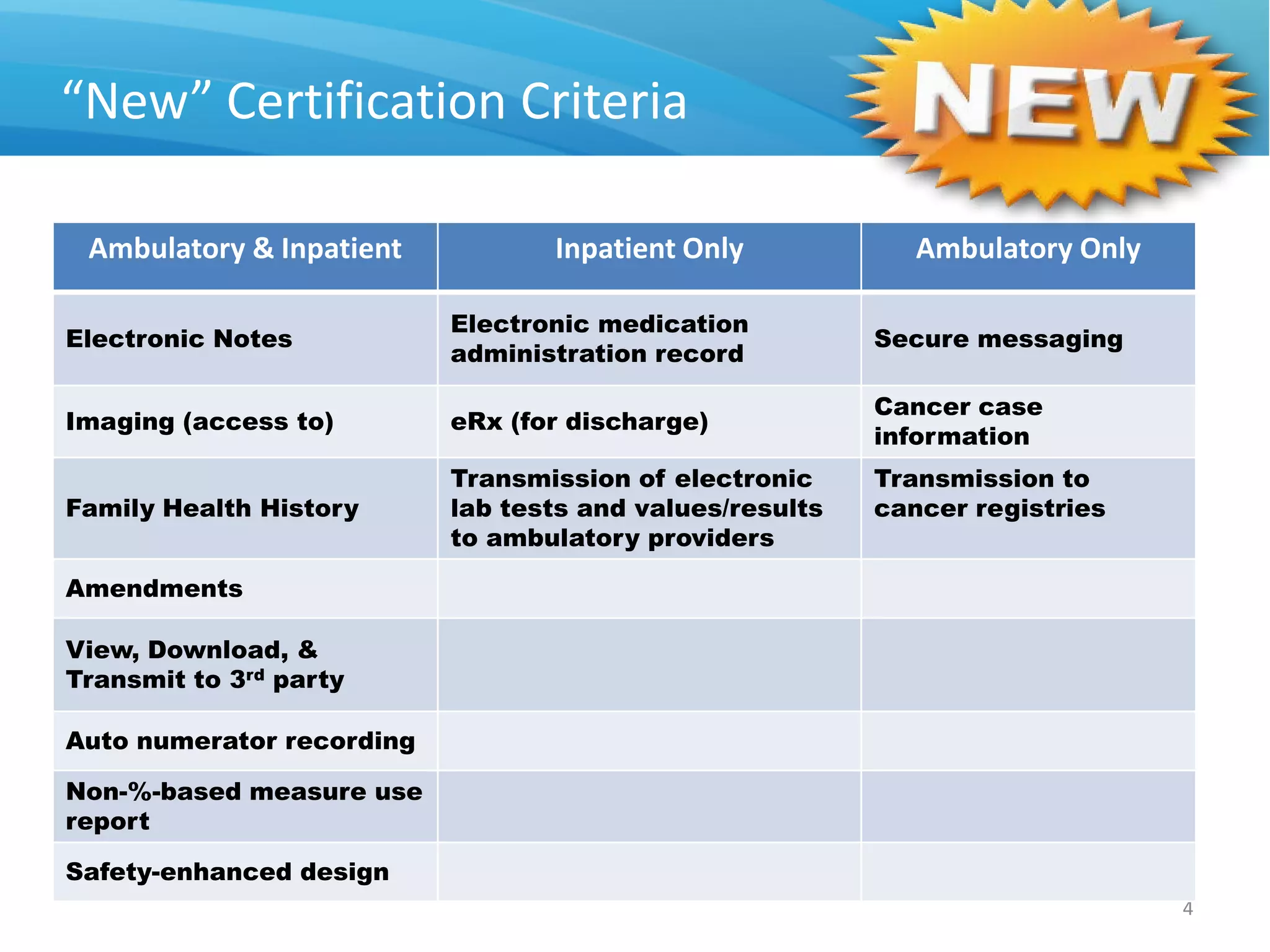
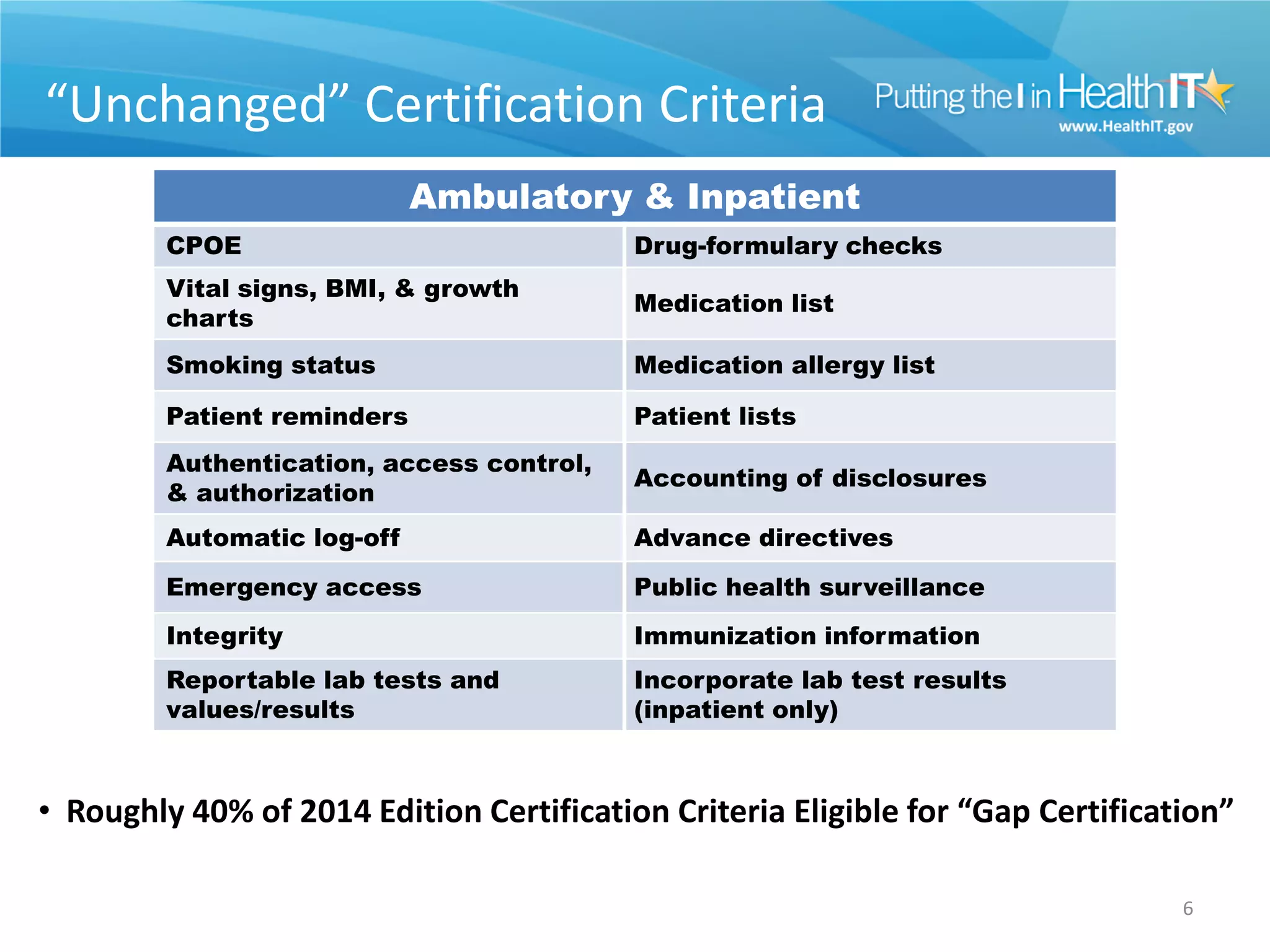
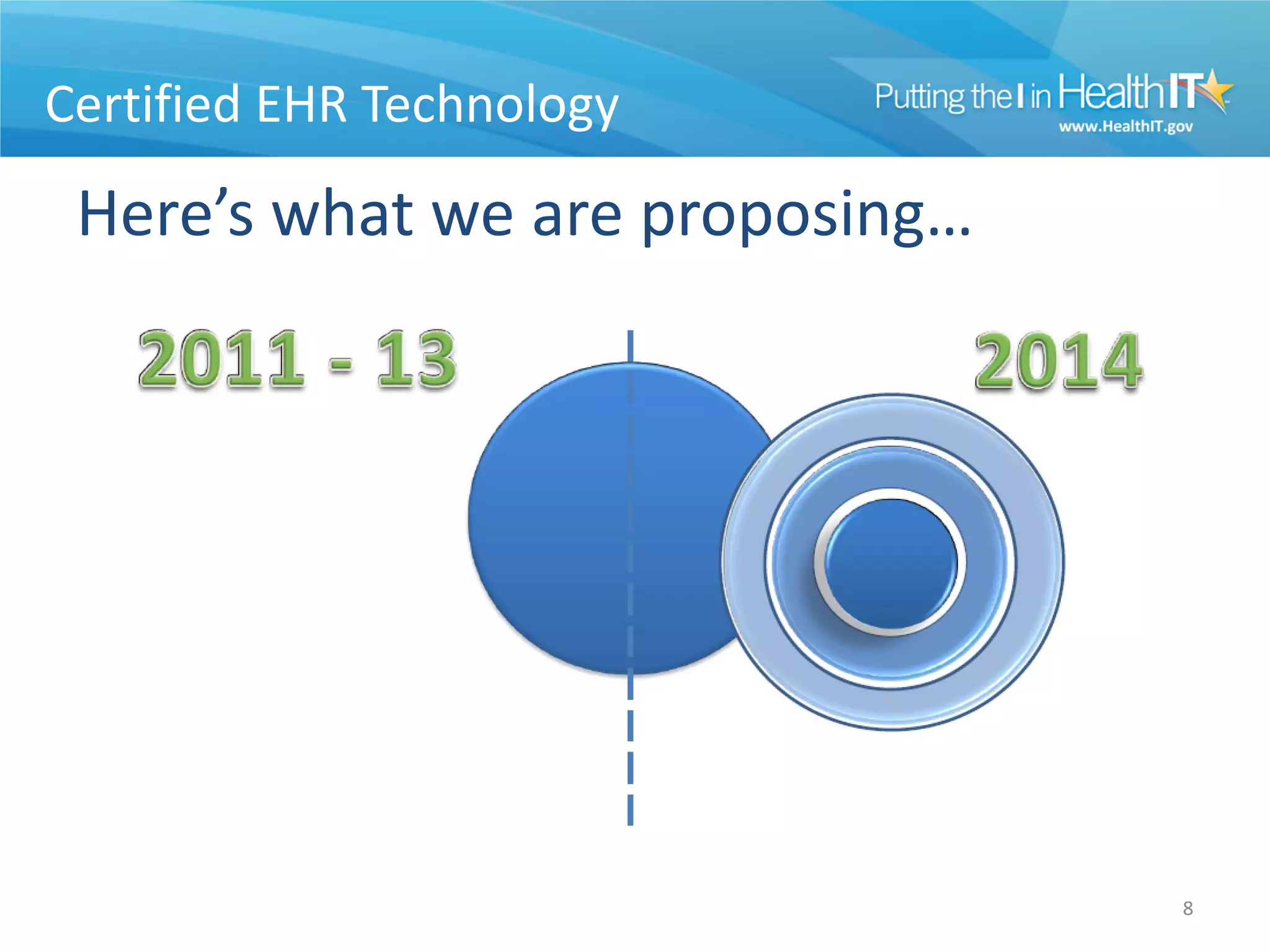
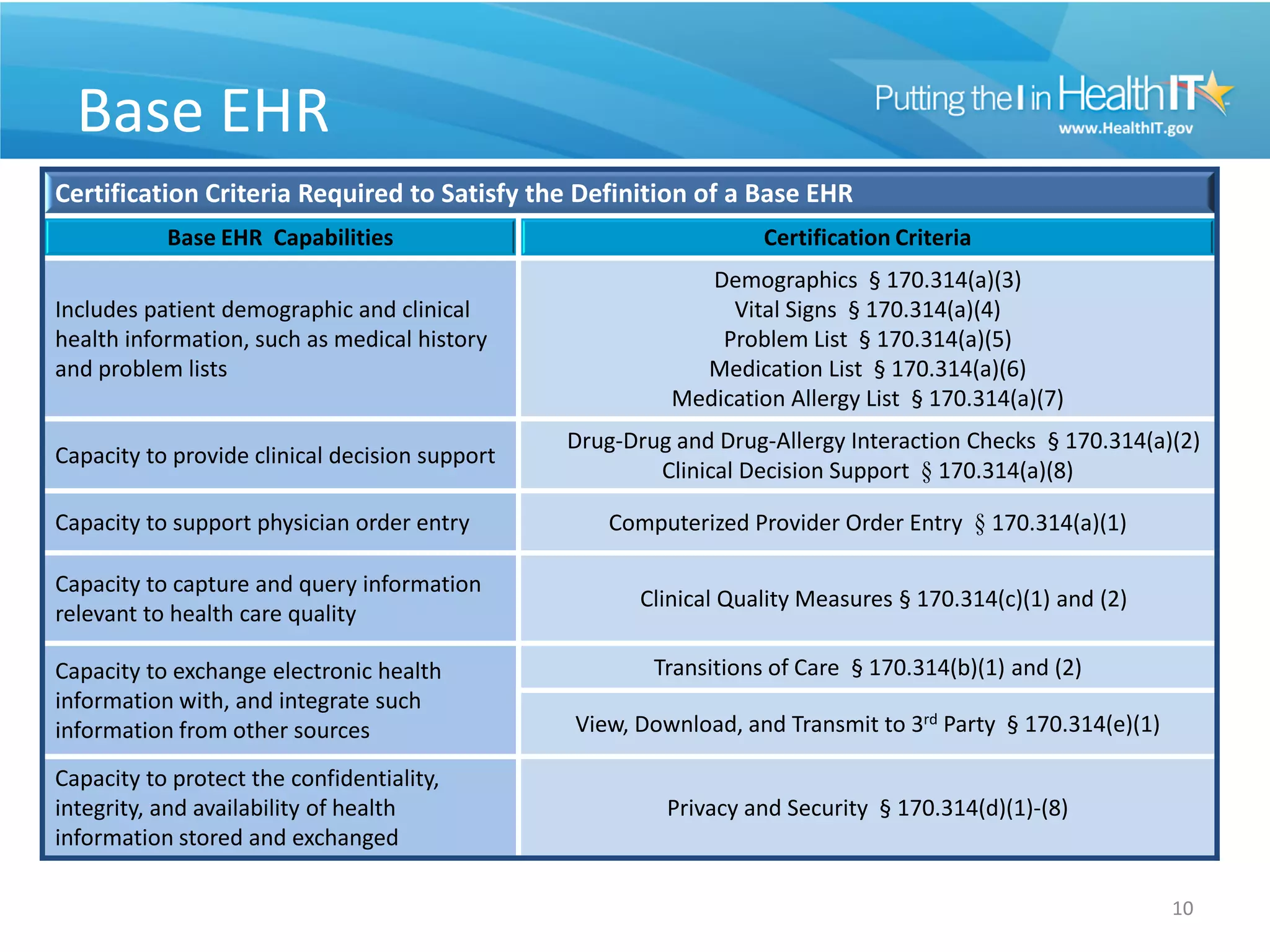
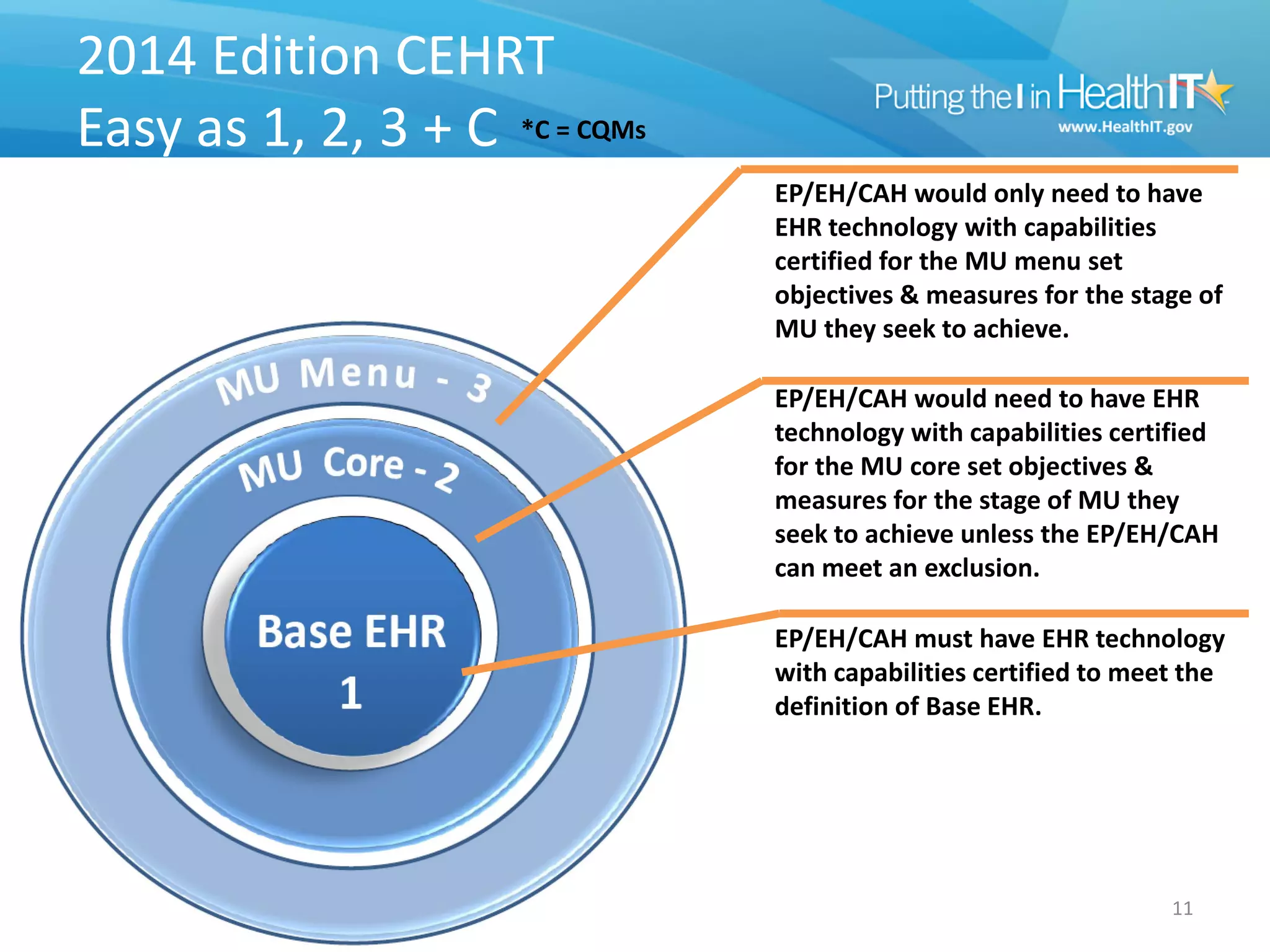
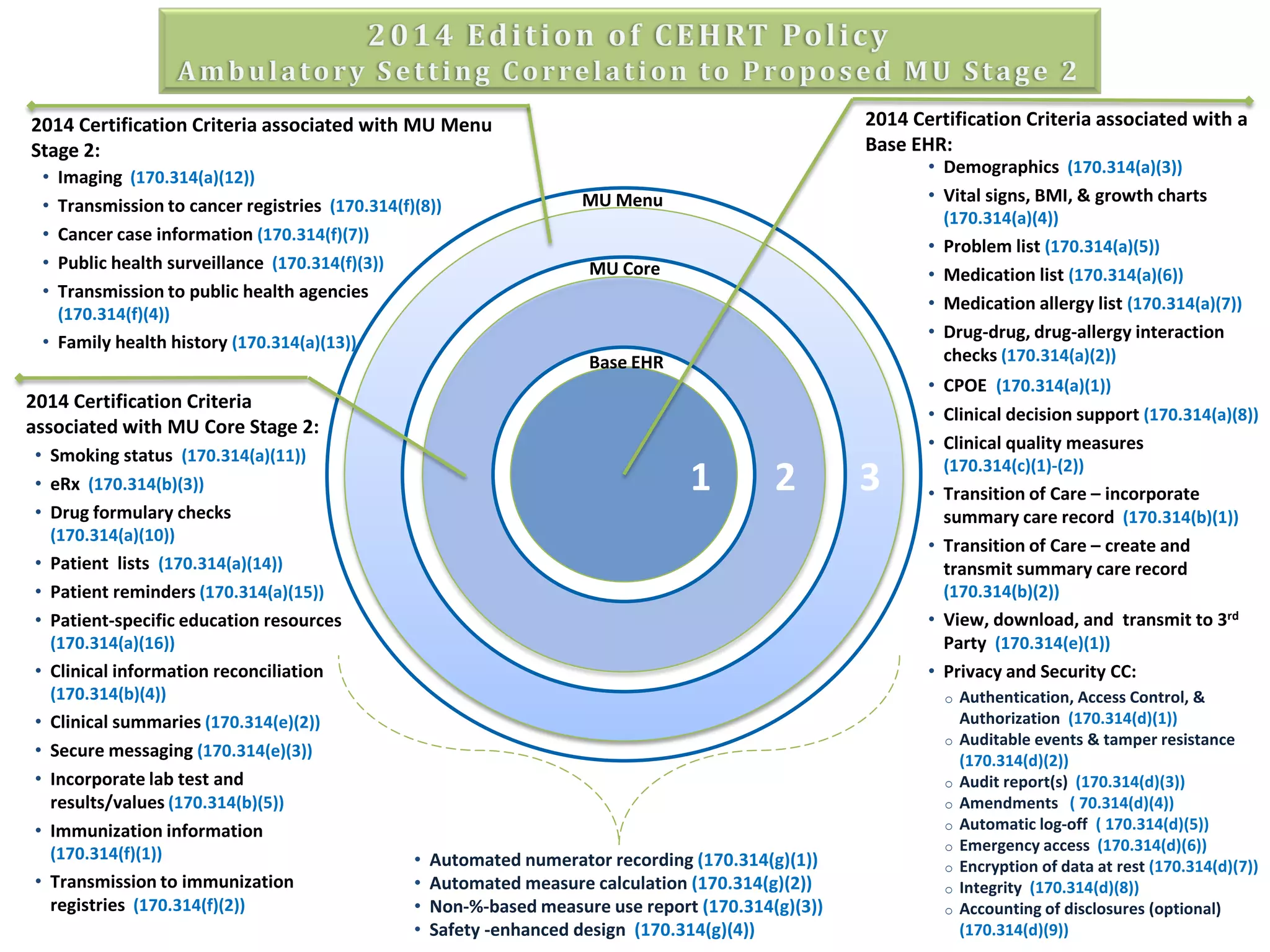
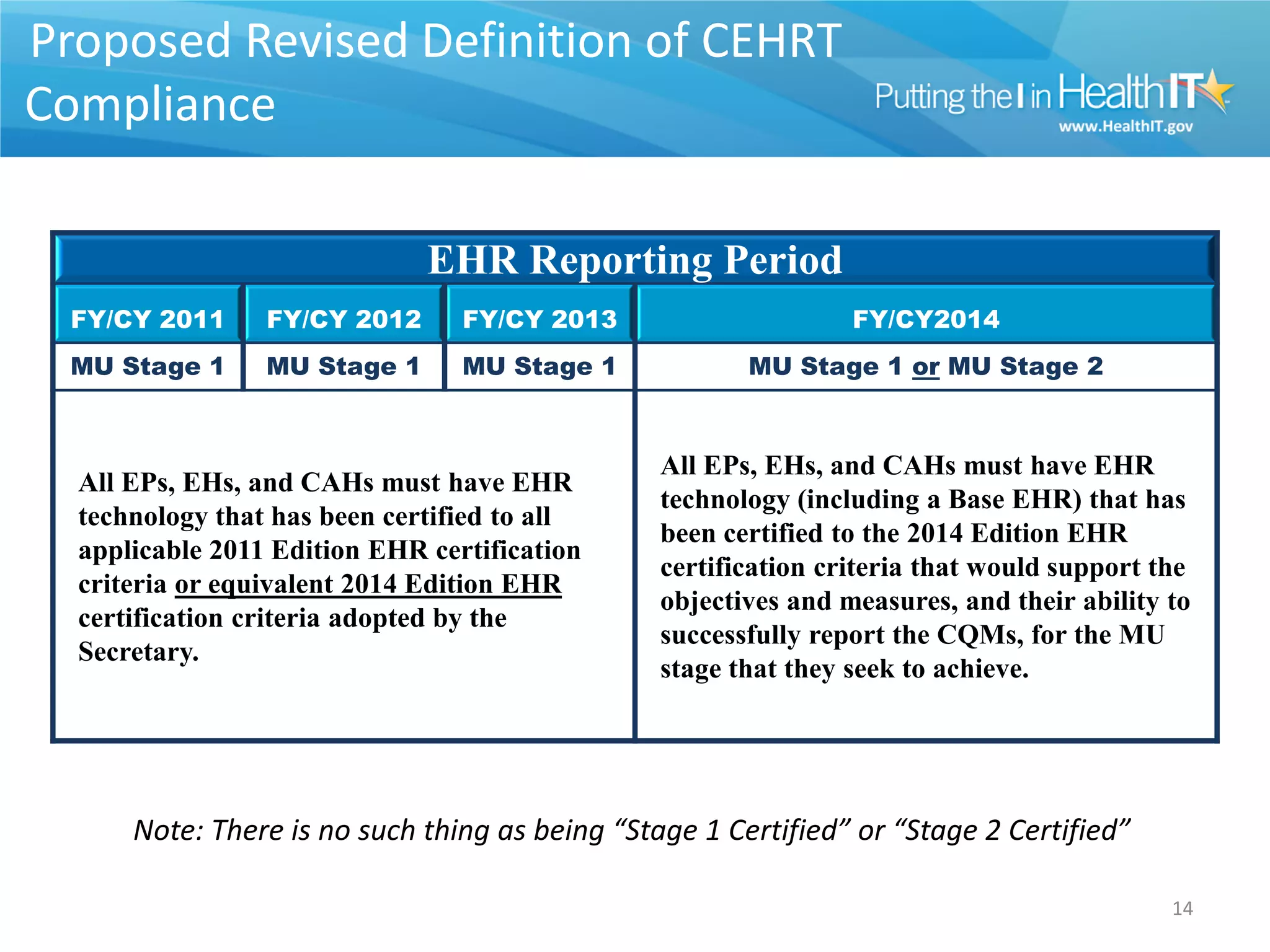
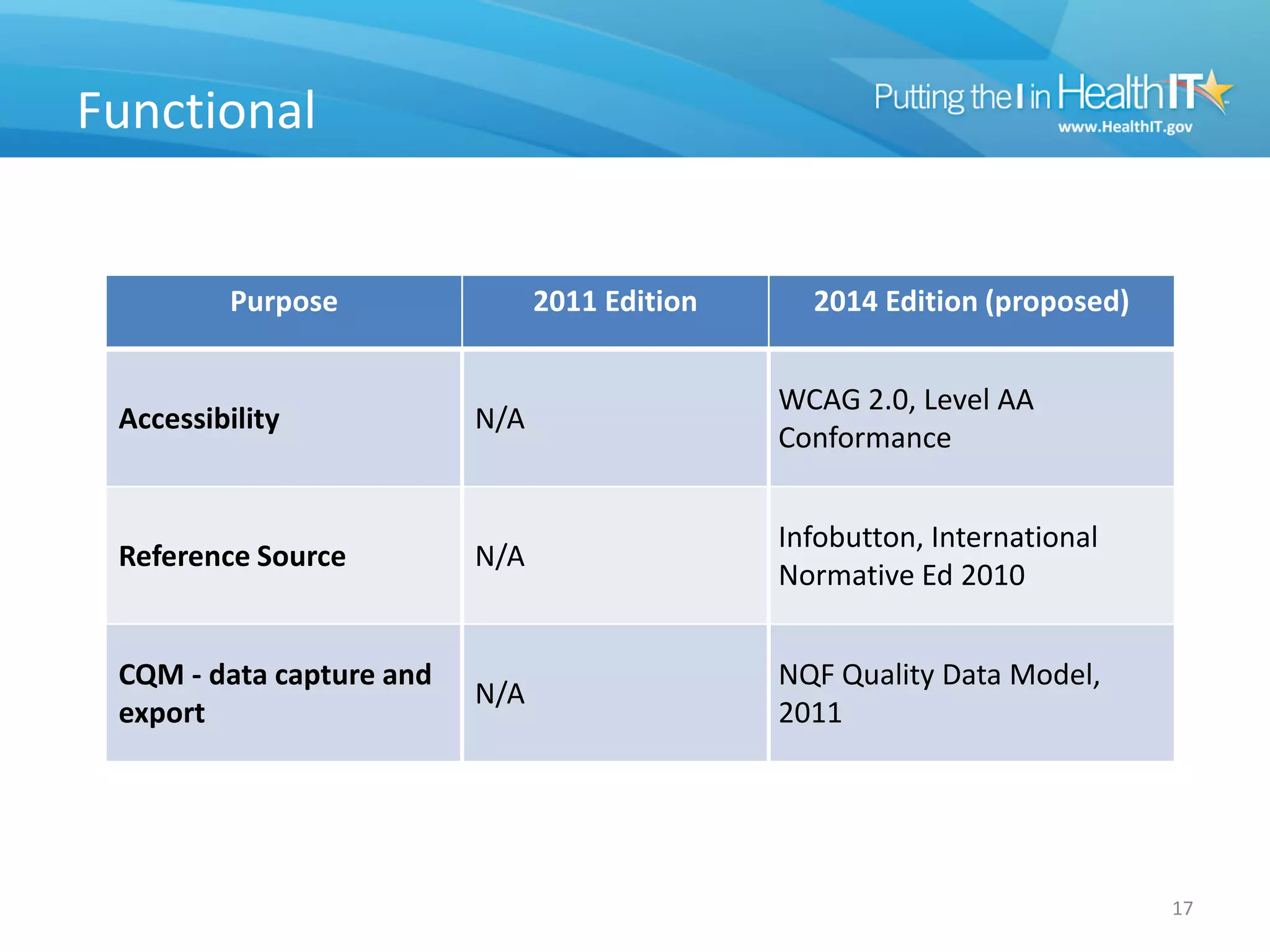
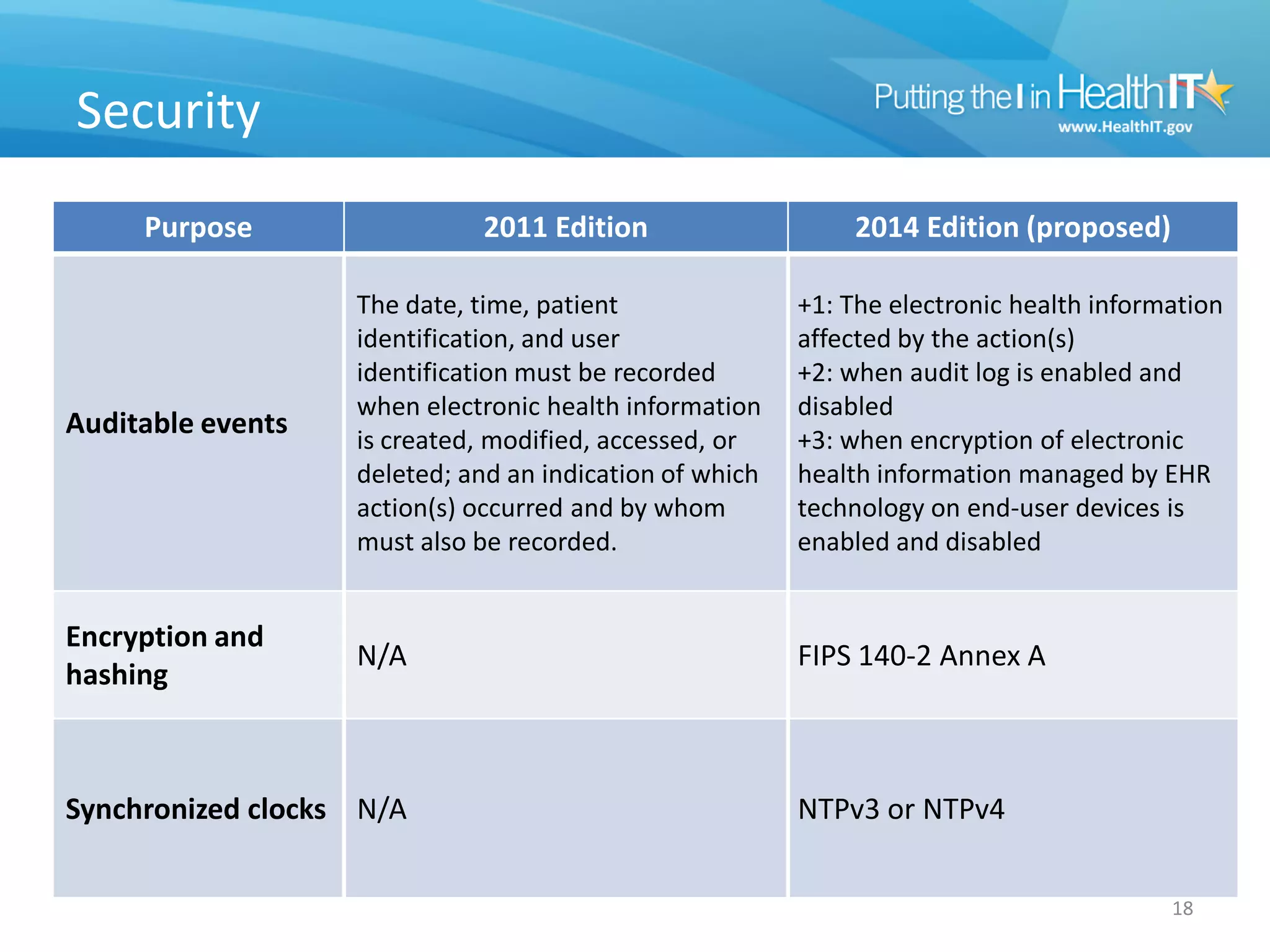
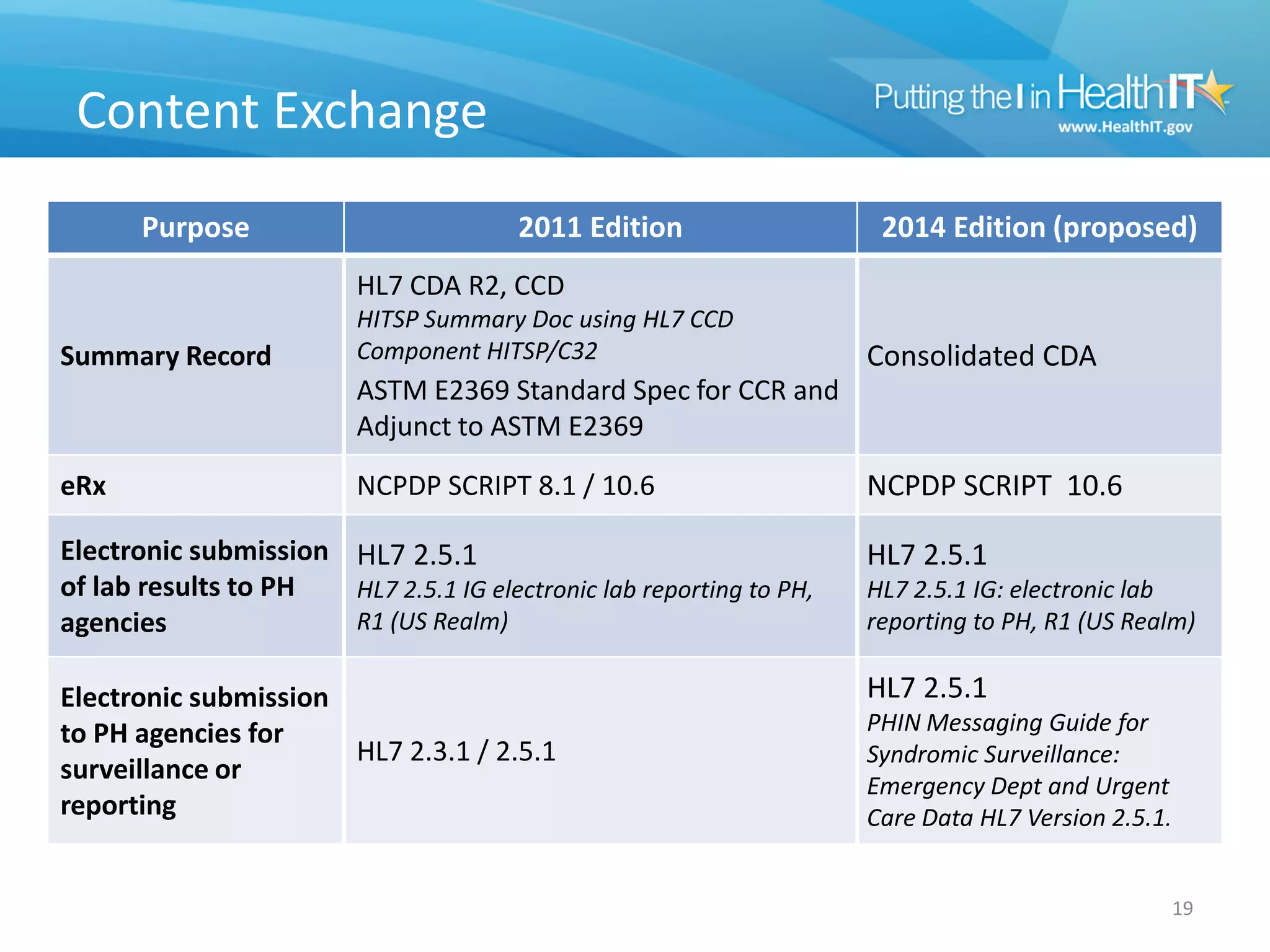
The proposed rule discusses changes to the 2014 Edition of certification criteria for electronic health records (EHRs). It proposes redefining certified EHR technology (CEHRT) as consisting of a base EHR plus additional certification criteria associated with meaningful use core and menu objectives. The base EHR would include criteria like patient demographics and clinical information, clinical decision support, and exchanging health information. Providers would need to meet the base EHR definition plus certification criteria for meaningful use core objectives unless an exclusion applies.










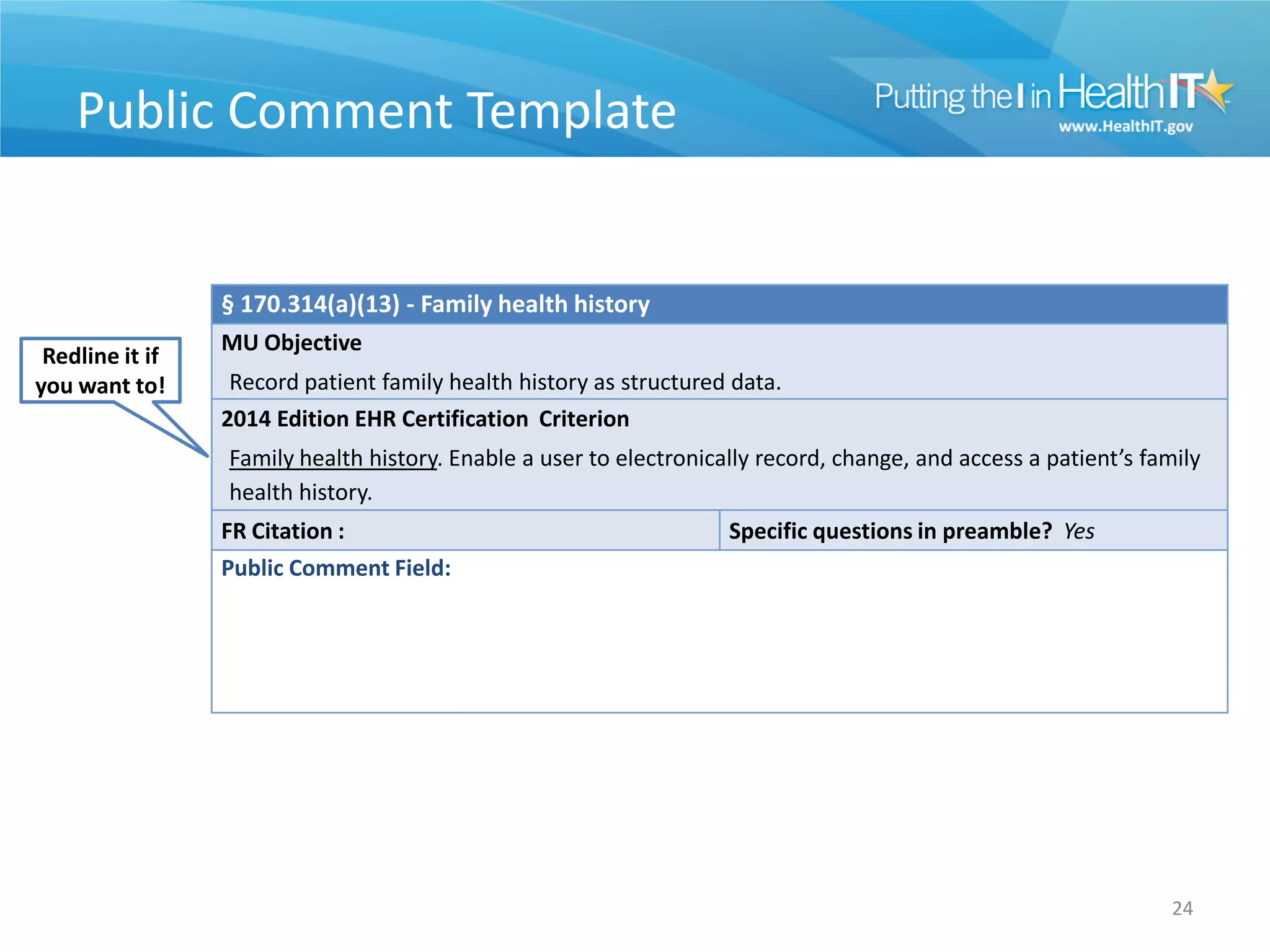
![Useful Information
• Enhancing the public comment experience
– Word version posted
– Comment template
– Other useful grids/materials
• 3 ways to comment
– Mail (snail/express)
– Electronic through regulations.gov [preferred]
– Hand deliver
23](https://image.slidesharecdn.com/2014sccedition2014hitsc022912-120302092821-phpapp02/75/2014-Standards-and-Certification-Criteria-2014-Edition-24-2048.jpg)