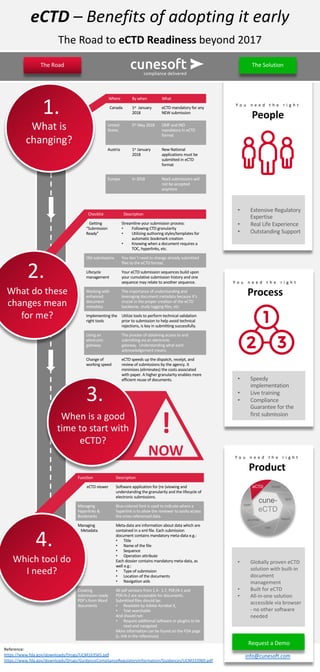
The document outlines the benefits and readiness requirements for adopting the electronic Common Technical Document (eCTD) format, highlighting regulatory deadlines for various regions in 2018. It emphasizes the importance of having the right expertise, processes, and tools to ensure compliance and efficient submissions. Additionally, it provides insights into managing metadata, creating submission-ready documents, and utilizing an electronic gateway for submissions.