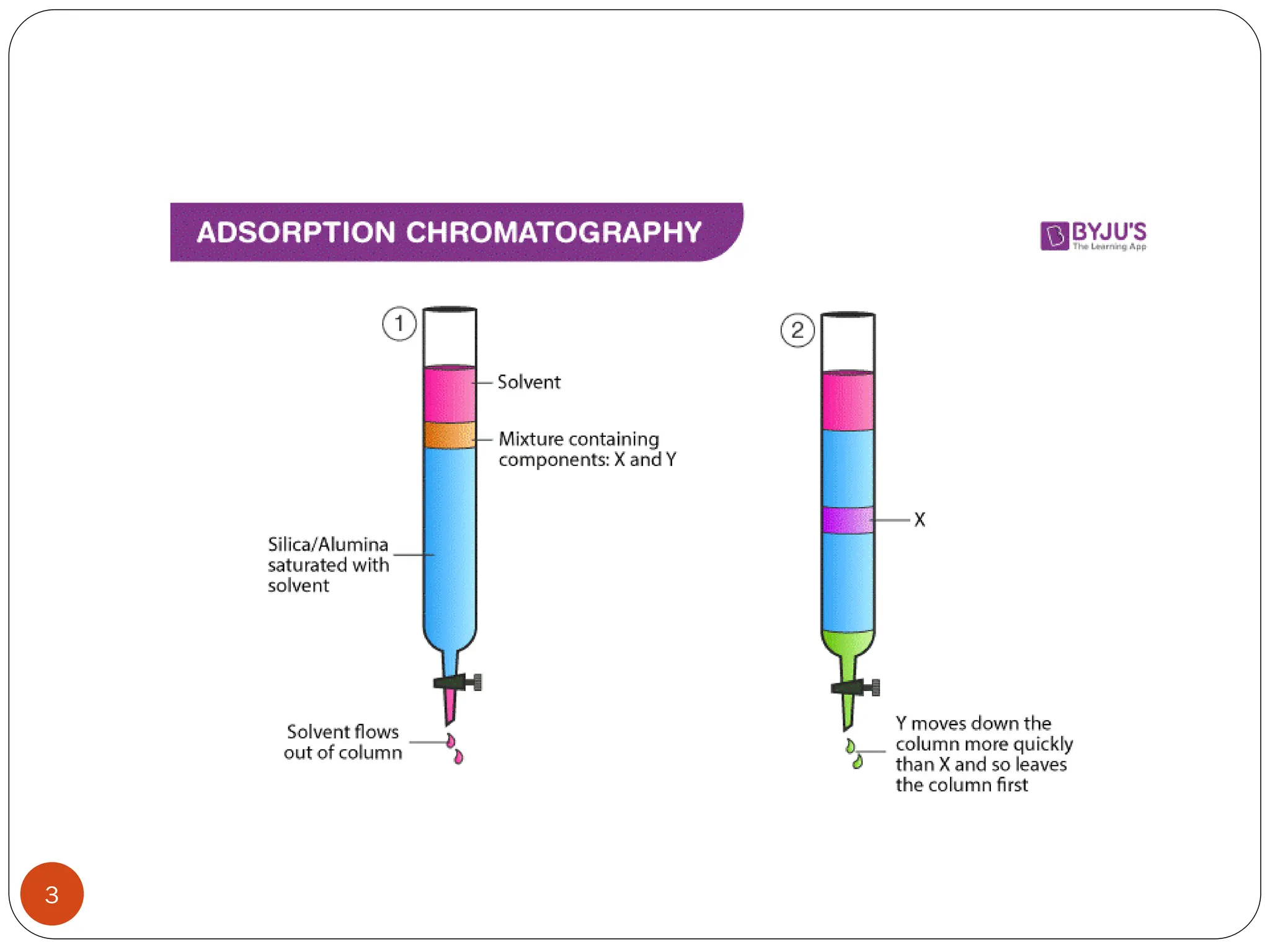
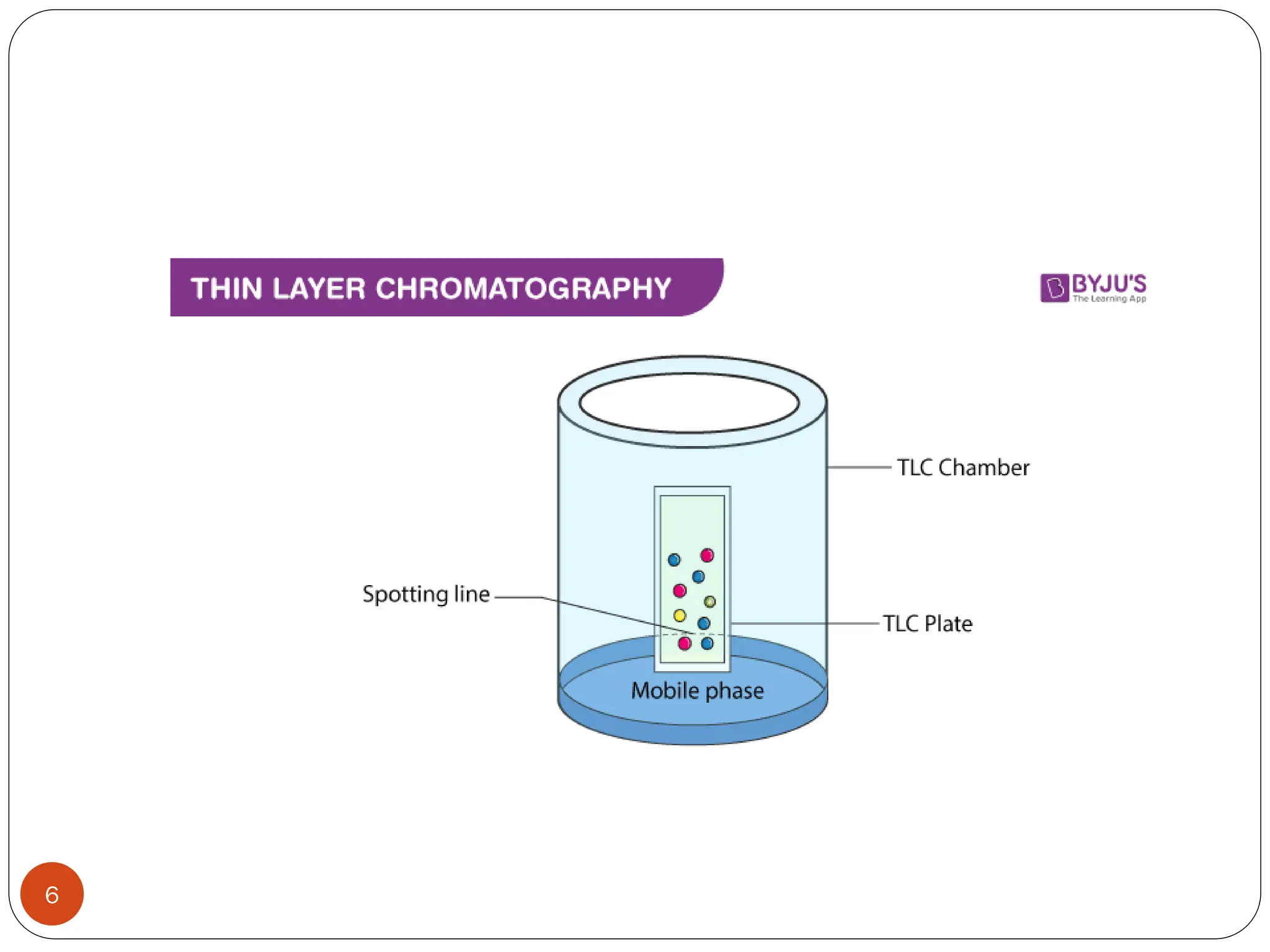
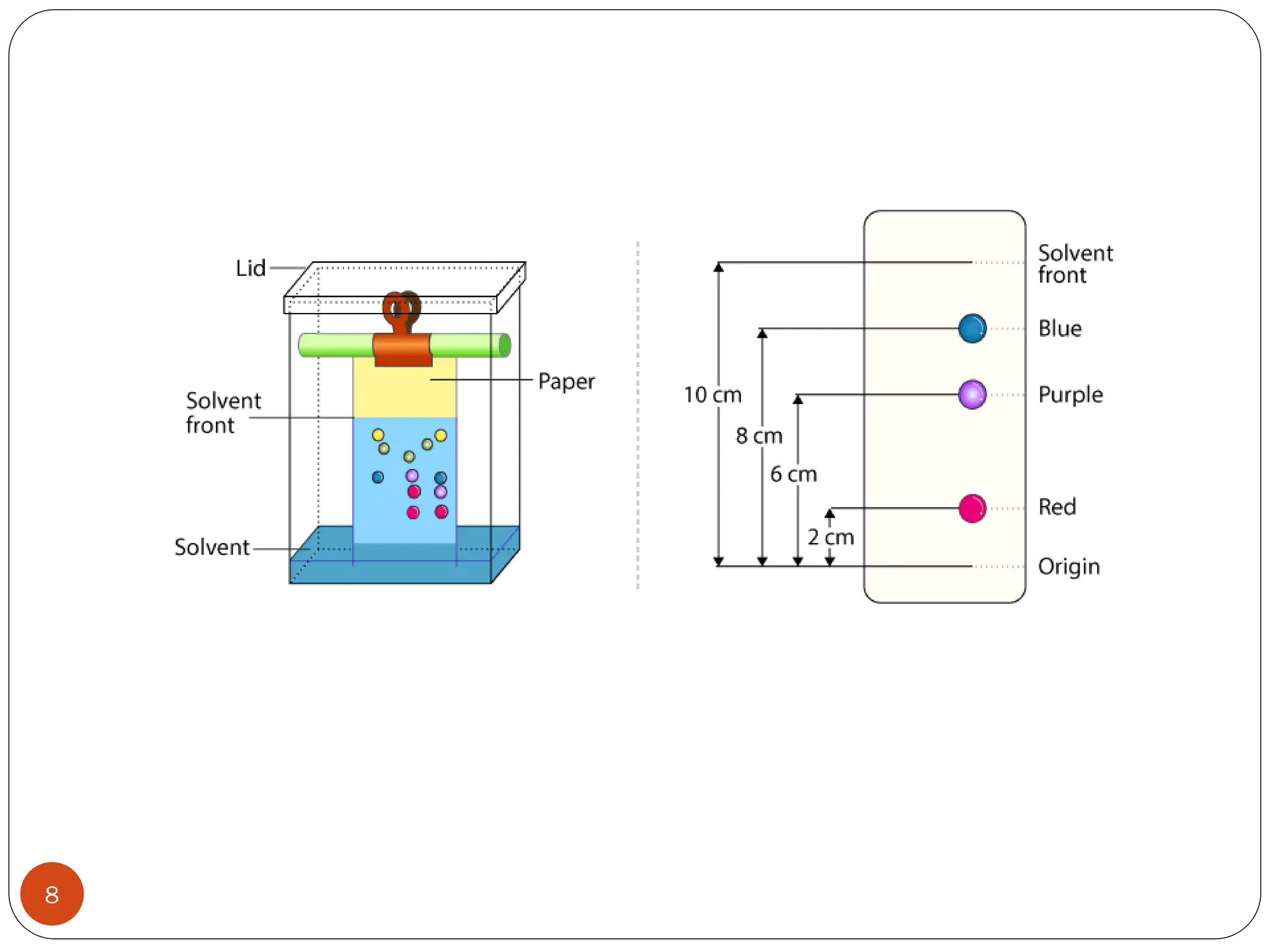
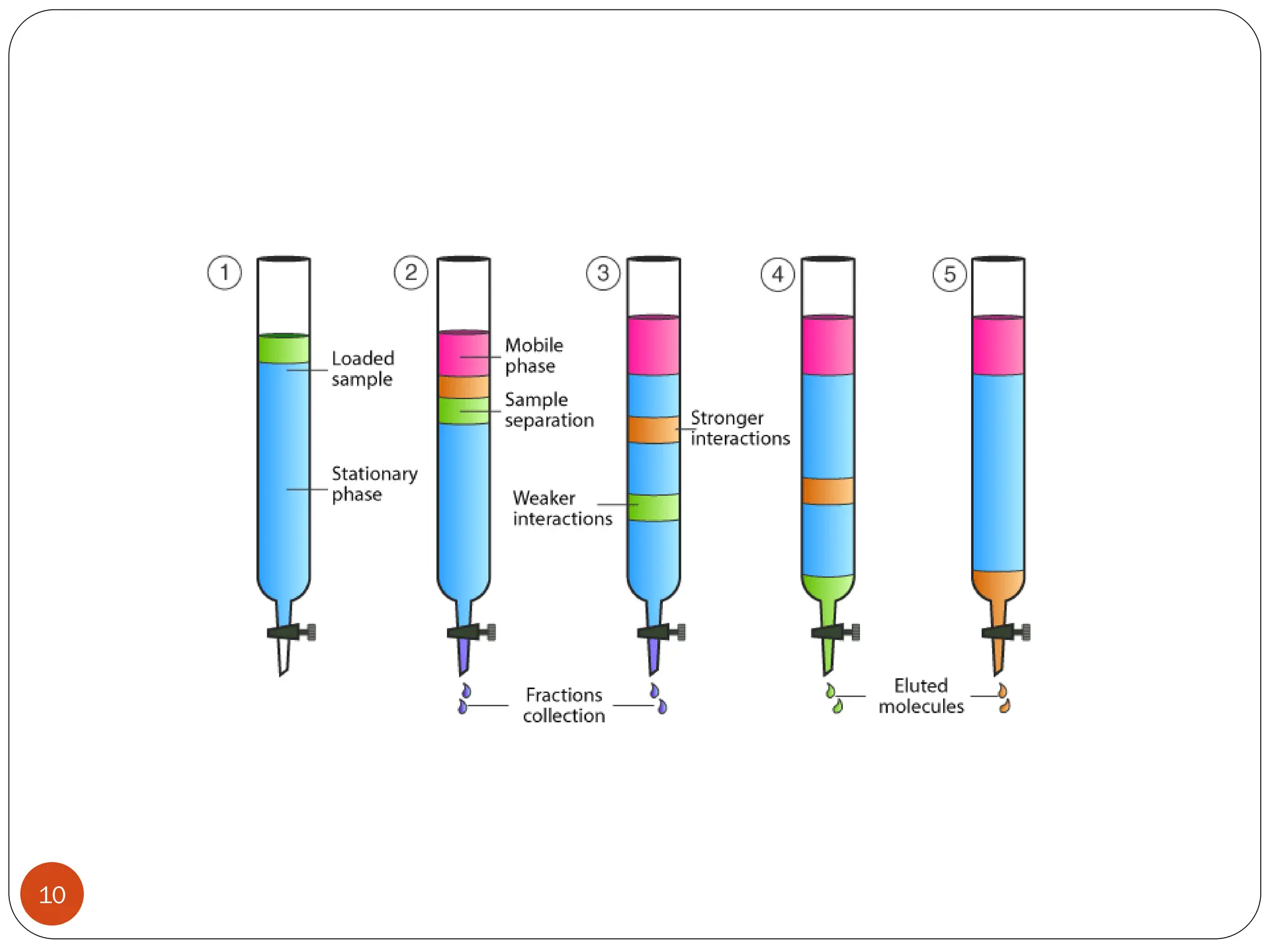
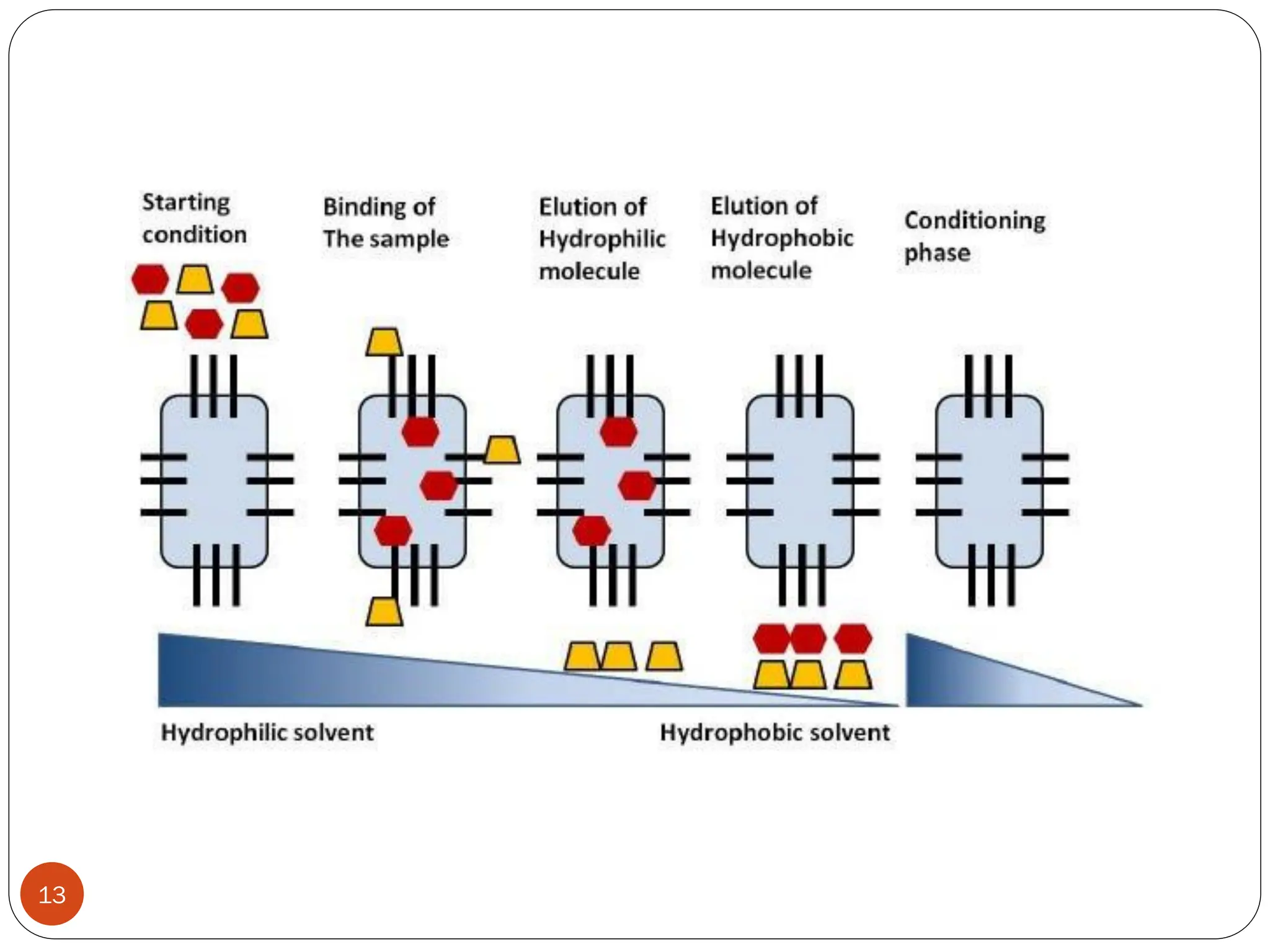
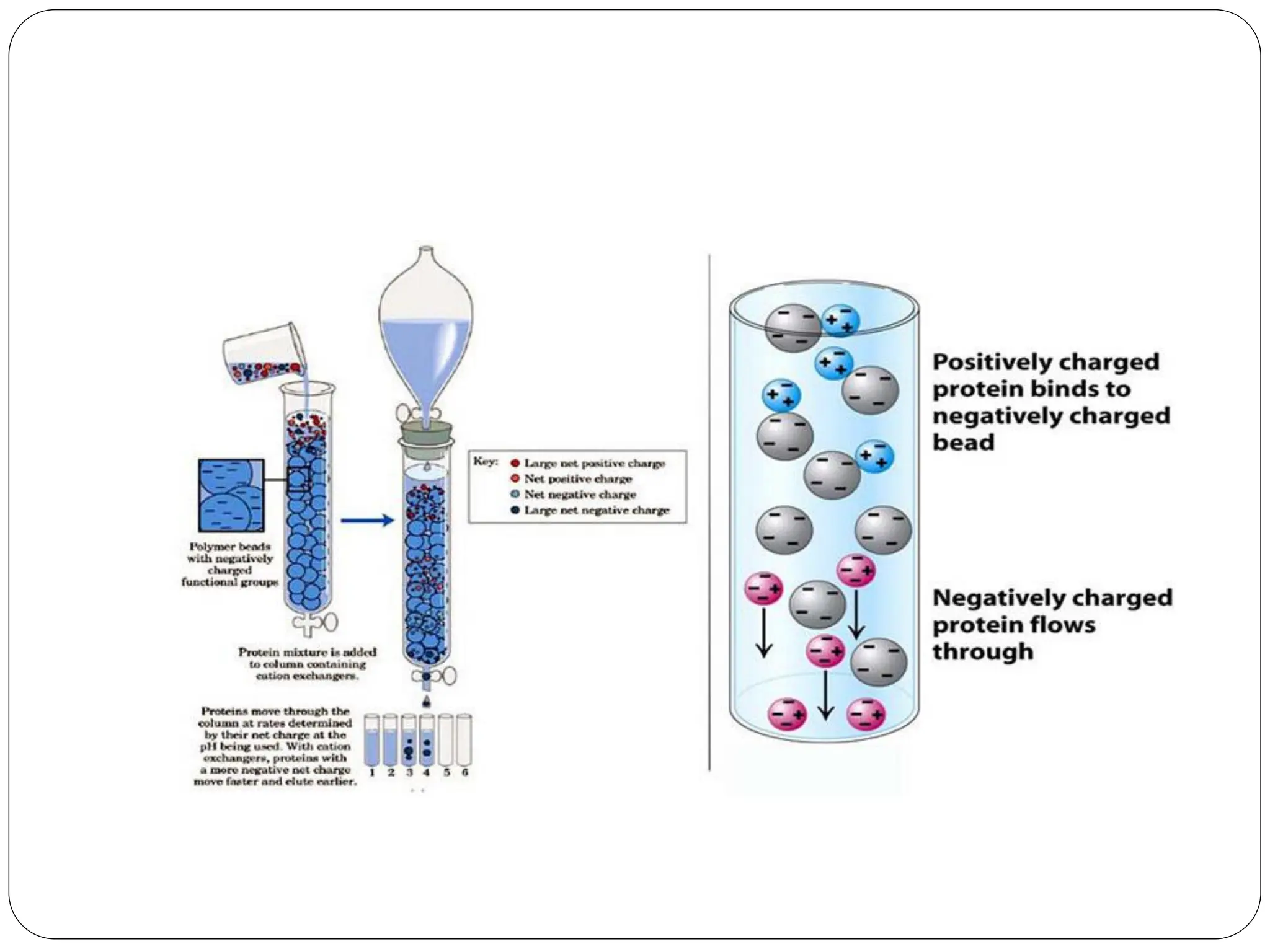
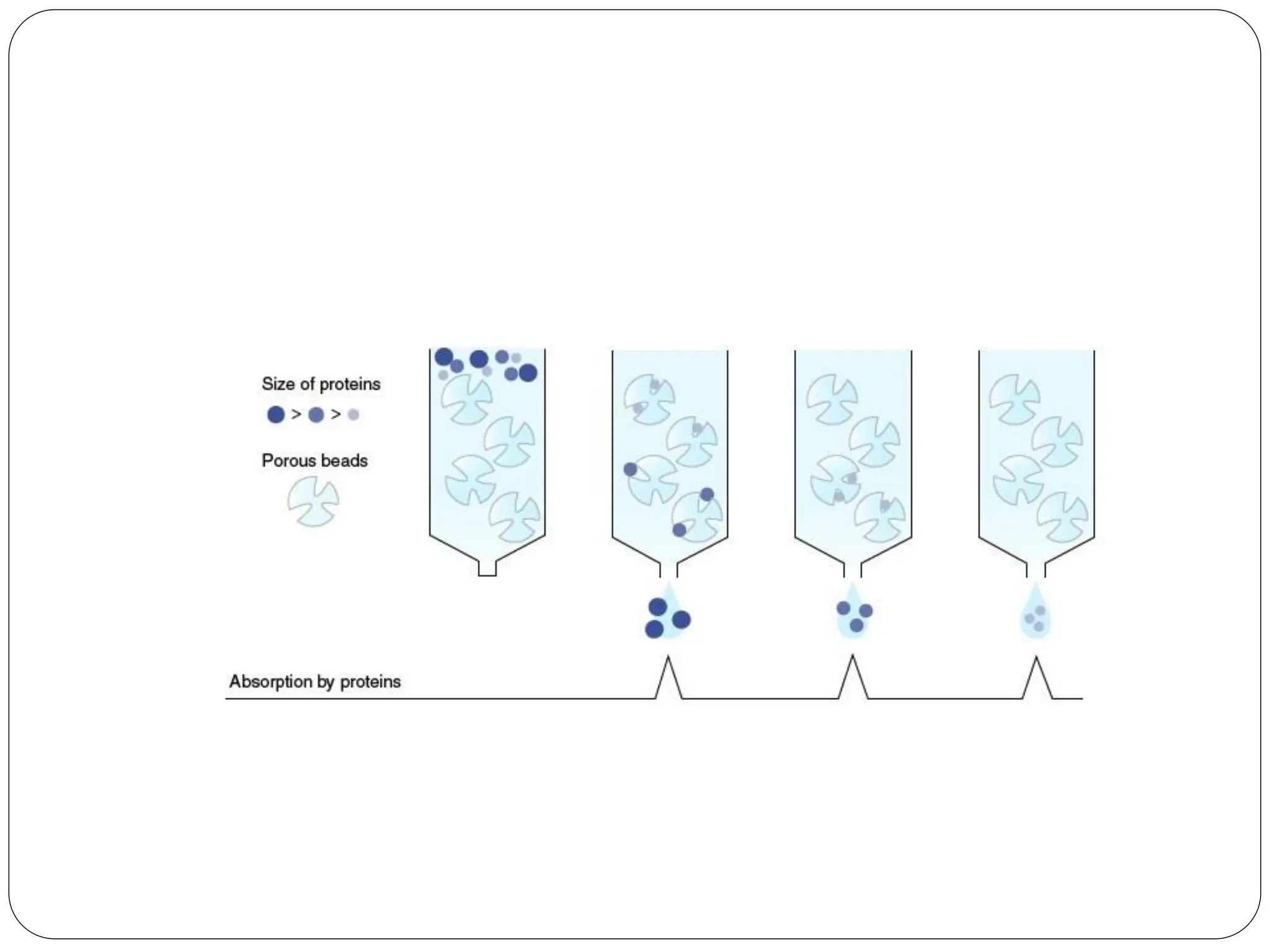
This document discusses various types of adsorption chromatography techniques including thin layer chromatography, paper chromatography, column chromatography, and gas-solid chromatography. It also describes reverse phase chromatography, ion exchange chromatography, and size exclusion chromatography. The key principles and applications of each technique are summarized.