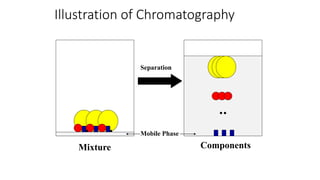
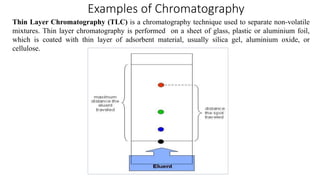
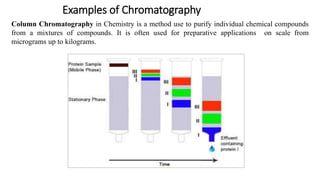
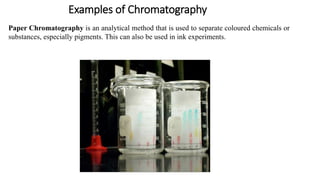
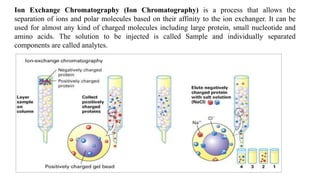
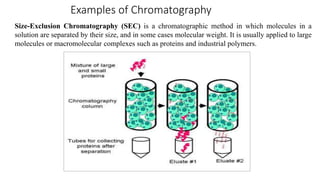
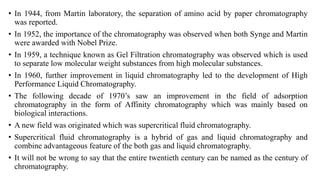
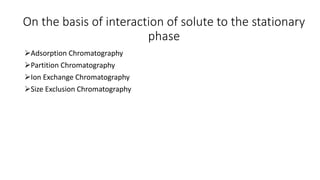
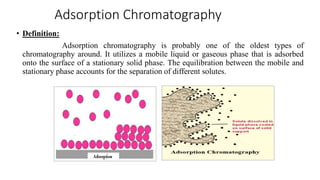
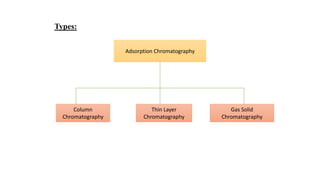
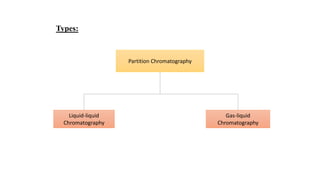
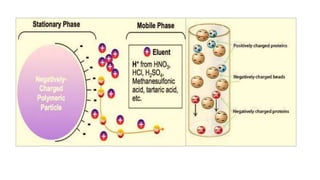
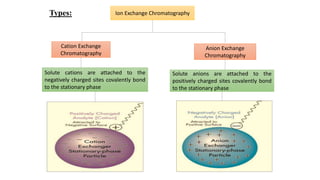
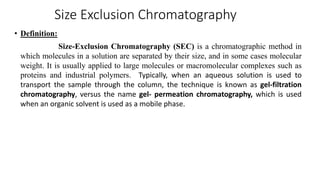
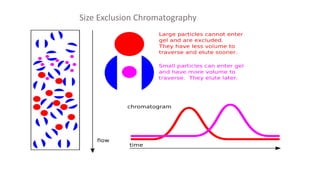
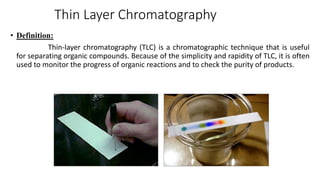
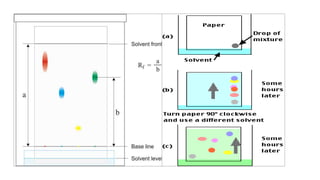
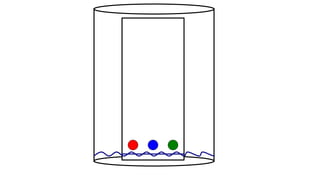
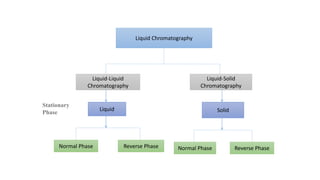
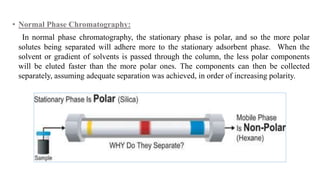
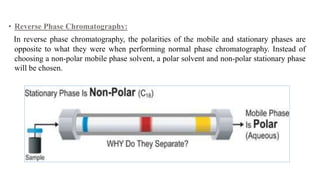
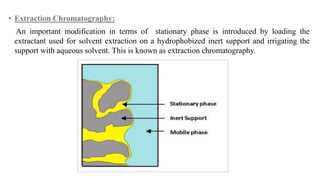
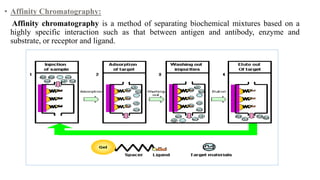
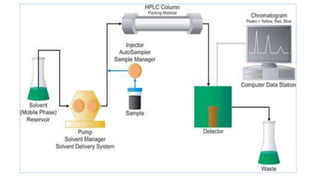
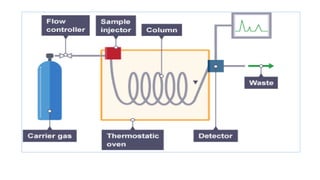
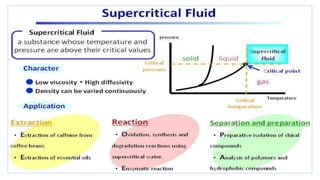
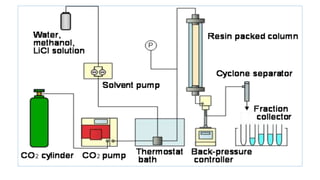
Chromatography is a separation technique involving a stationary phase and a mobile phase, allowing components to redistribute based on their differing affinities. Various forms of chromatography, such as thin layer, column, paper, ion exchange, size exclusion, and high performance liquid chromatography, are employed for different applications including pigment separation, protein purification, and analysis of chemical mixtures. Historical advancements since its introduction in 1906 highlight its evolution, leading to specialized techniques like supercritical fluid chromatography and affinity chromatography.