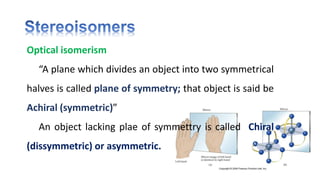
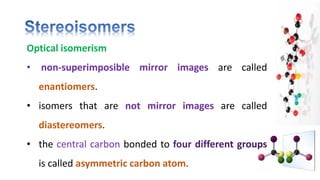
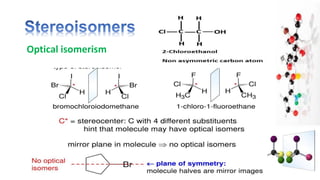
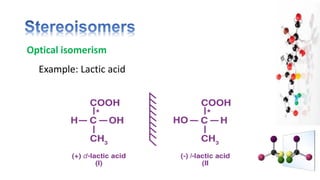
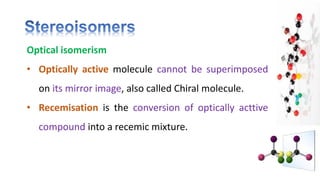
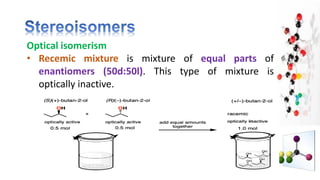
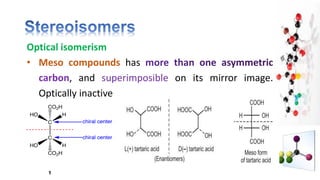
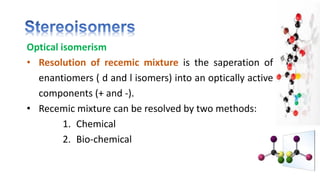
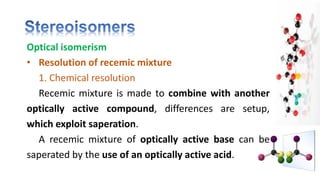
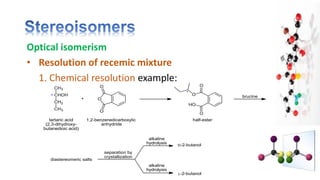
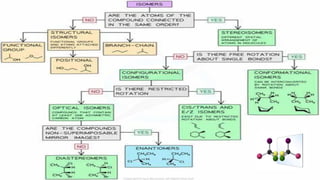
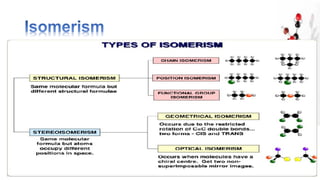
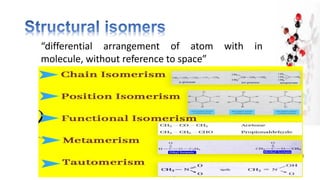
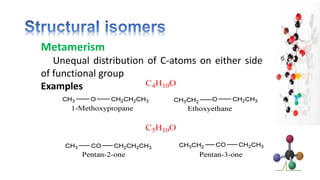
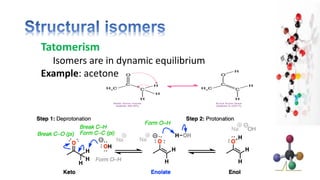
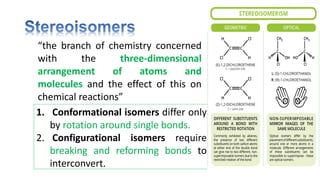
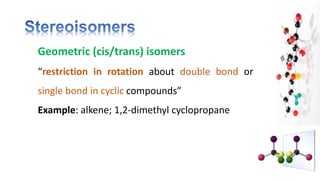
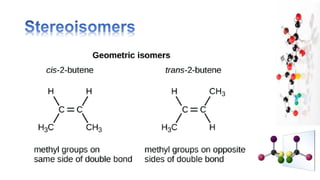
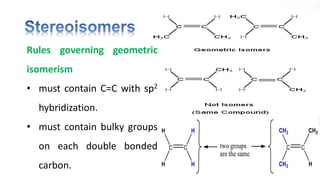
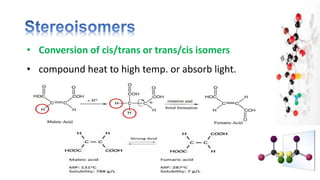
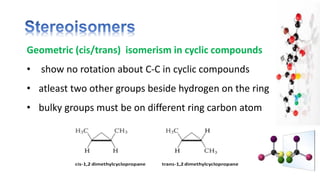
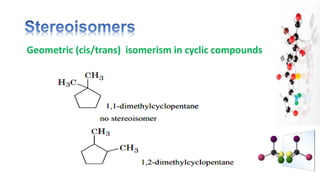
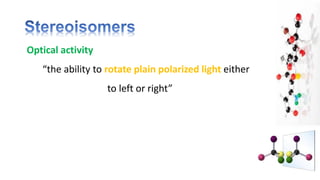
The document discusses various aspects of stereochemistry, including isomerism types such as metamerism, tautomerism, geometric isomerism, and optical isomerism. It explains the concepts of conformational and configurational isomers, outlines the conditions for geometric isomerism, and explores optical activity and the significance of chiral molecules. Additionally, it details methods for resolving racemic mixtures into optically active components.




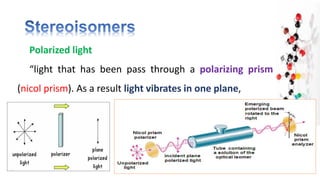
![Polarized light
• The polarimeter measures the rotation of plane
polarized-light, that has passed through a solution.
• Rotation, in degree is, [α]
• Right rotation = Clockwise [+] = dextrorotatory (d)
• Left rotation = Anticlockwise [-] = levorotatory (l)](https://image.slidesharecdn.com/documentfromvengeance-240425160817-5b6eddf4/85/Document-from-Vengeance-pdfbbbbbbjjjjjjj-19-320.jpg)