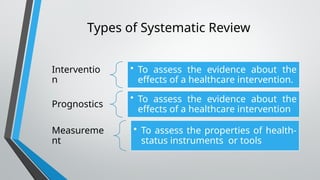
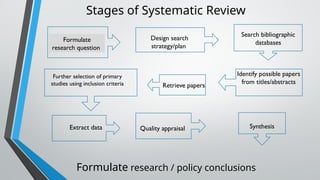
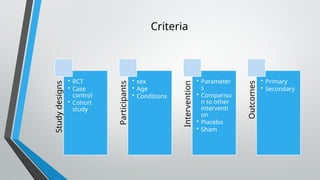
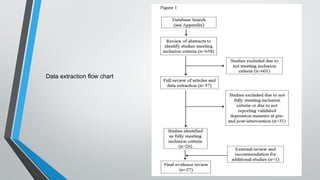
This document outlines the development of a systematic review, defining it as a structured analysis of evidence pertaining to a specific research question. It emphasizes the reasons for conducting systematic reviews, including supporting evidence-based practice and informing clinical policy, while detailing stages such as formulating research questions and evaluating study design. The document also provides guidance on creating a research strategy, identifying data sources, and assessing methodological quality.