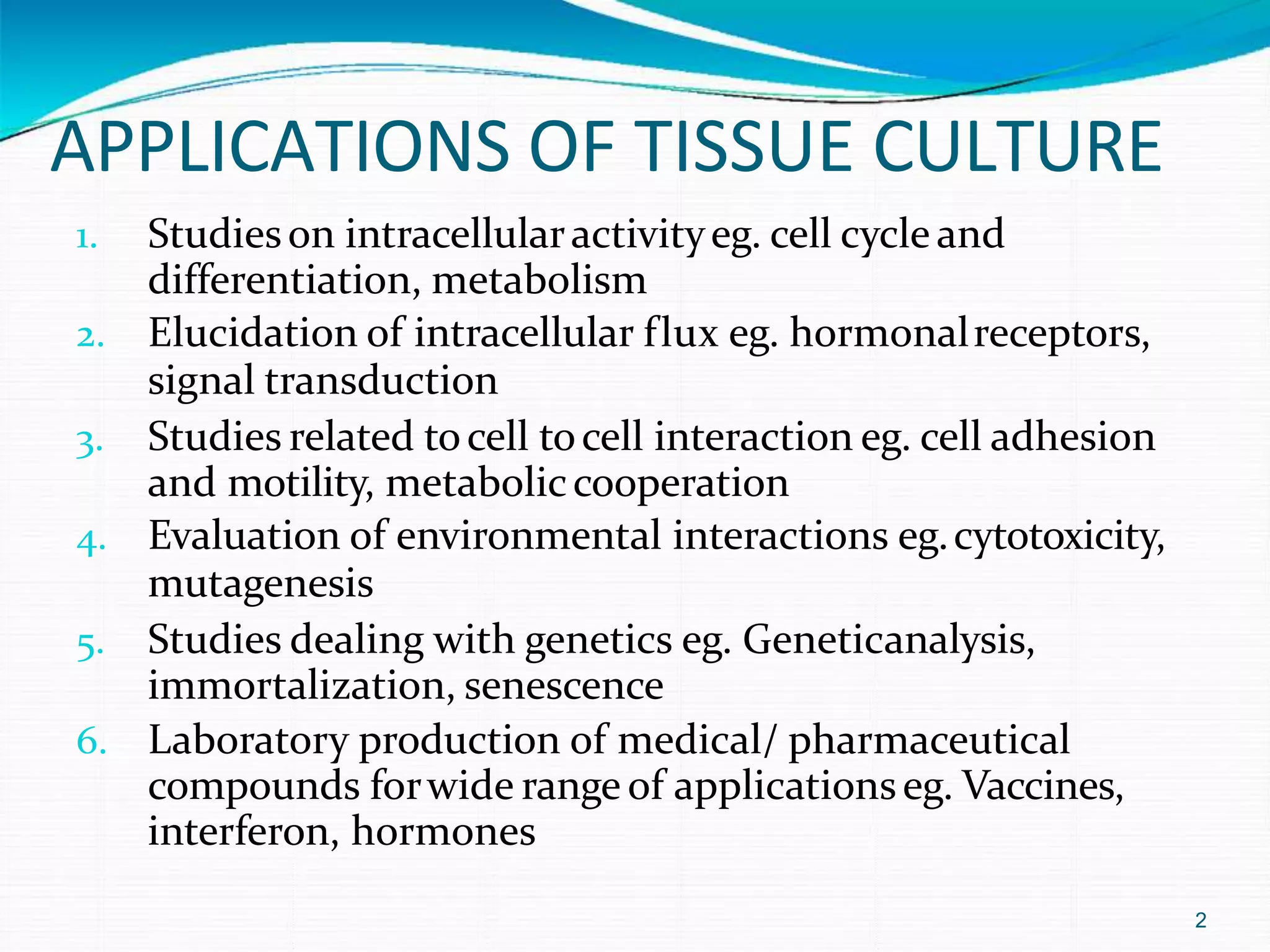
The document details the principles and applications of tissue culture techniques, highlighting their uses in studying intracellular activity, environmental interactions, and the laboratory production of pharmaceutical compounds. It outlines the development of cell culture technology, including the use of antibiotics and defined media, and provides instructions for preparing and maintaining culture media as well as guidelines for subculturing cells. Additionally, it discusses contamination types, their effects on cell cultures, and methods for detecting such contaminants.









![11
Decontaminate external surfaces of all vials, including the medium bottle, with ethanol or
isopropanol.
To formulate Endothelial Growth Medium (EGM™ Medium), transfer the contents of the
EGM™ SingleQuots™ Kit (Lonza Catalog No. CC-4133 containing Bovine Brain Extract [BBE];
Ascorbic Acid, Hydrocortisone, Epidermal Growth Factor [hEGF], Fetal Bovine Serum [FBS]
and Gentamicin/Amphotericin-B [GA]) to EBM™ Basal Medium with a pipette, and rinse
each vial with medium.
To formulate Microvascular Endothelial Growth Medium-2 (EGM™-2MV Medium), transfer
the contents of the EGM™-2MV SingleQuots™ Kit (Lonza Catalog No. CC-4147 containing
human Epidermal Growth Factor [hEGF], Vascular Endothelial Growth Factor [VEGF], R3-
Insulinlike Growth Factor-1 [R3-IGF-1], Ascorbic Acid, Hydrocortisone, human Fibroblast
Growth Factor-Beta [hFGF-β], Fetal Bovine Serum [FBS], and Gentamicin/Amphotericin-B
[GA]) to EBM™-2 Basal Medium with a pipette, and rinse each vial with medium.
NOTE: If there is concern that sterility was compromised during the supplementation process,
the entire newly prepared growth medium may be re-filtered with a 0.2 µm filter to assure
sterility. Routine re-filtration is not
Preparation of Culture Media](https://image.slidesharecdn.com/day1-ppttissueculture-210624114452/75/Day-1-ppt-tissue-culture-11-2048.jpg)














