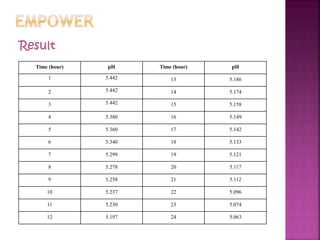
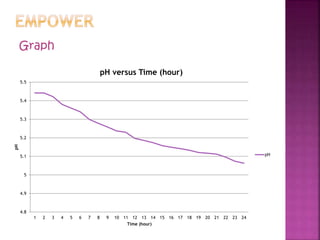
Bacterial fermentation causes milk to spoil over time. Lactic acid bacteria and coliforms are the main types of bacteria present in milk. When milk is left at room temperature for 1 day, its pH decreases steadily as the lactic acid bacteria ferment the milk's lactose into lactic acid, causing acidification. Monitoring the milk's pH drop over 24 hours showed the rate of change in pH remaining constant as fermentation occurred.