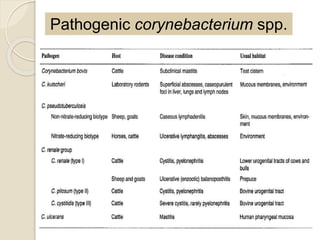
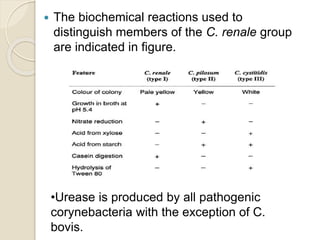
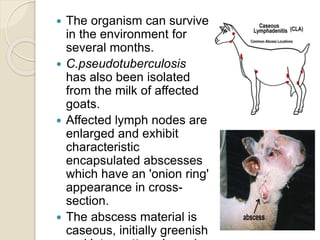
This document summarizes coryneform bacteria, including their classification and characteristics. It focuses on the genus Corynebacterium, describing several pathogenic species and the diseases they cause. Corynebacterium pseudotuberculosis causes ulcerative lymphangitis in horses and caseous lymphadenitis in sheep and goats. Corynebacterium renale can lead to bovine pyelonephritis and ulcerative balanoposthitis in sheep. Identification involves examining colony morphology, biochemical reactions, and isolating bacteria from lesions.