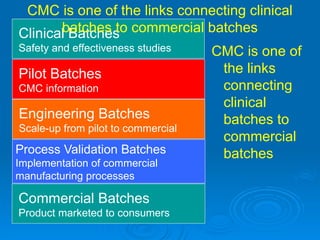
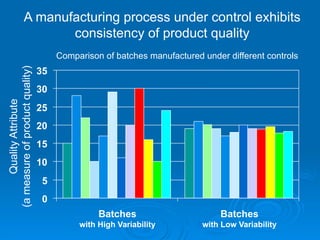
The document outlines the significance of Chemistry, Manufacturing, and Controls (CMC) and Good Manufacturing Practices (GMPs) in ensuring that marketed drugs maintain the same quality attributes as those demonstrated to be safe and effective in clinical studies. It emphasizes that CMC establishes connections between clinical and commercial drug batches, underscoring the importance of process understanding and consistent quality control throughout manufacturing. The document also details the role of regulatory bodies in monitoring compliance and maintaining product quality post-approval.