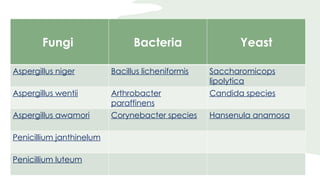
The document discusses the industrial production of citric acid, highlighting its importance, production methods, and applications. Citric acid is primarily produced through fermentation using strains like Aspergillus niger, with a focus on optimizing growth conditions and recovery processes. The conclusion emphasizes the wide applications of citric acid, with annual production exceeding 1 million tons.