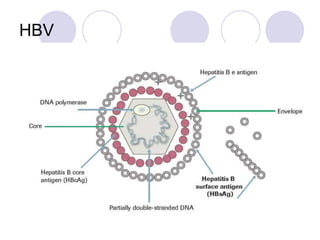
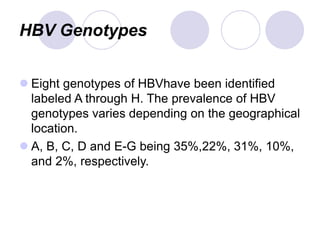
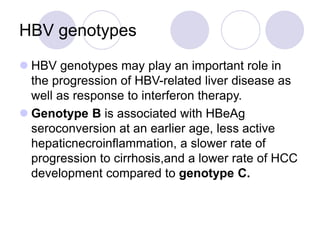
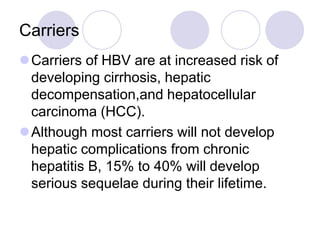
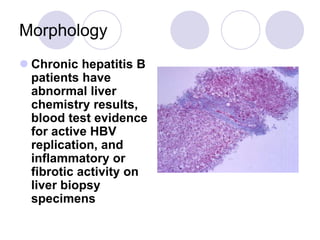
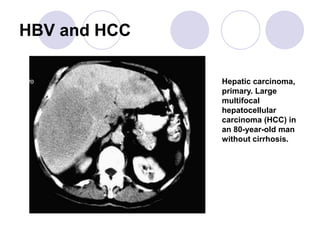
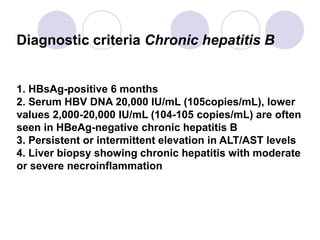
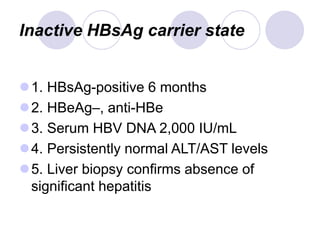
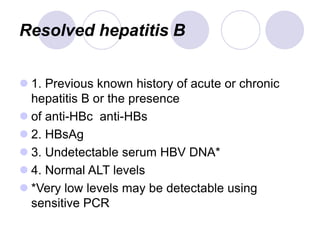
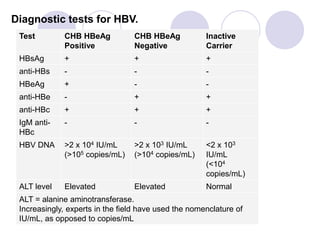
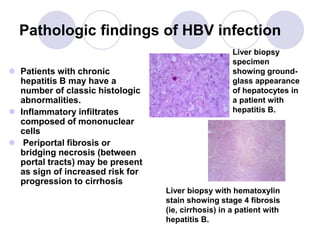
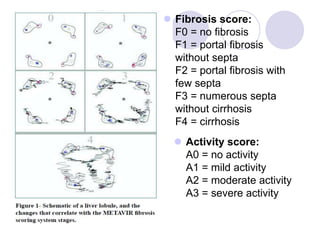
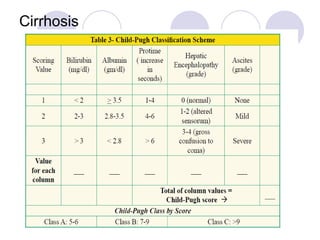
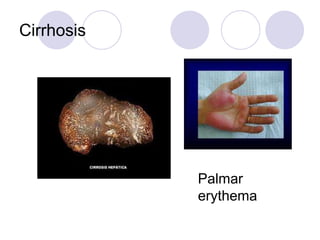
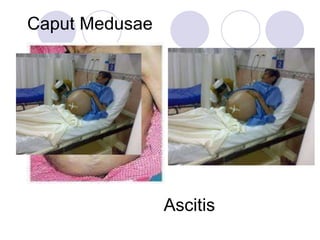
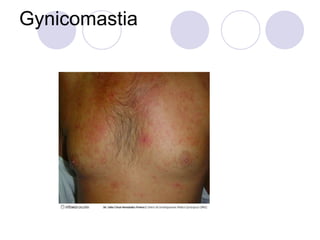
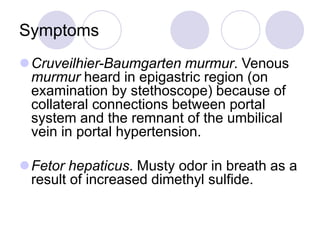
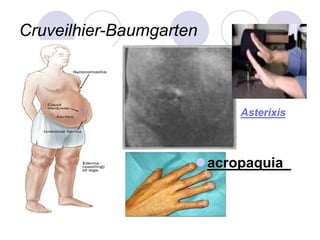
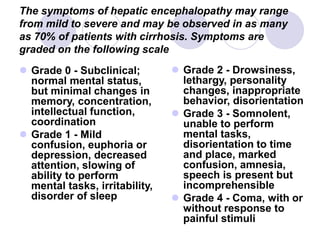
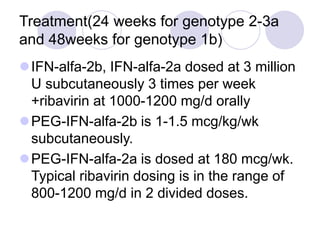
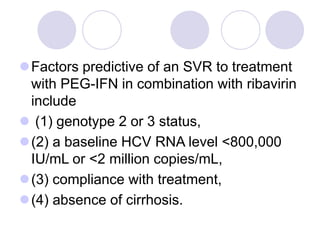
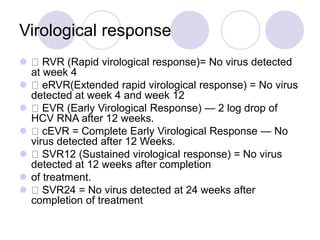
Chronic viral hepatitis can be caused by hepatitis B or hepatitis C virus infections lasting more than 6 months. It often presents with non-specific symptoms but can lead to serious complications affecting the liver and other organs. Treatment aims to suppress viral replication through medications like interferons or oral antivirals in order to prevent progression to cirrhosis or liver cancer. Accurate diagnosis involves identifying viral markers in the blood and seeing inflammatory changes on liver biopsy.