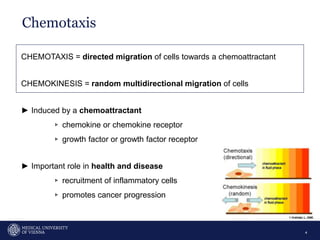
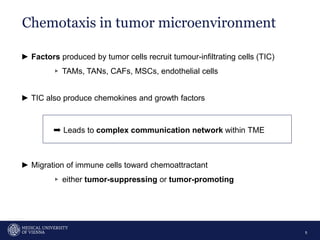
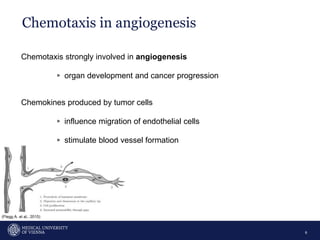
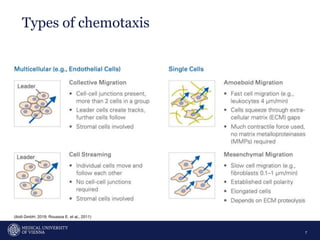
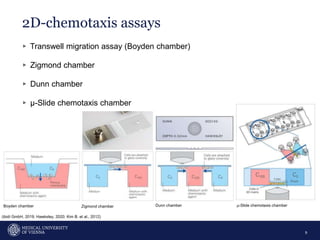
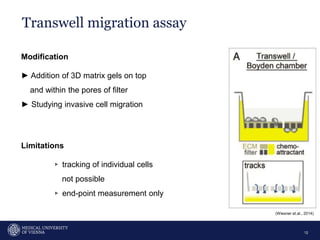
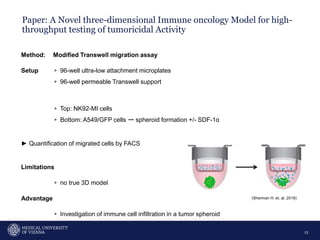
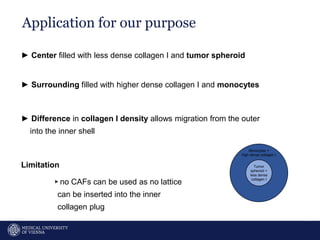
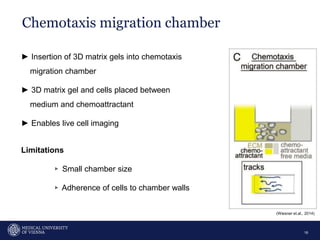
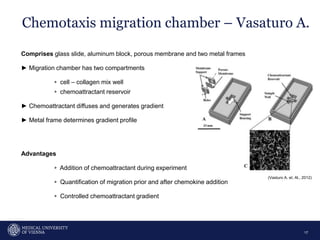
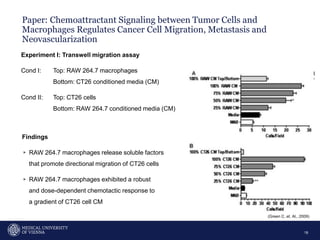
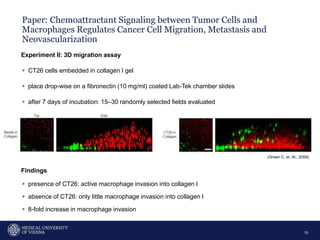
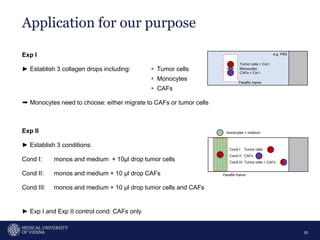
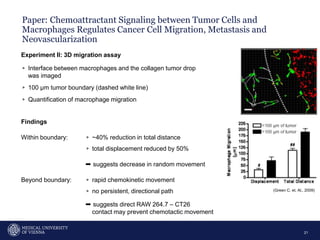
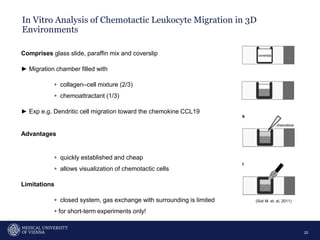
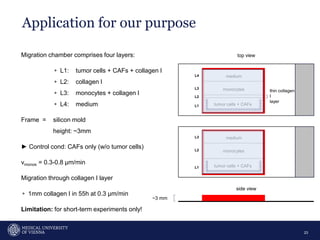
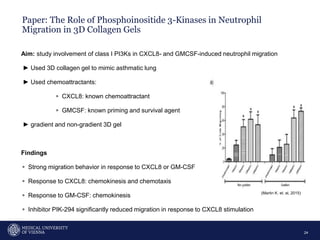
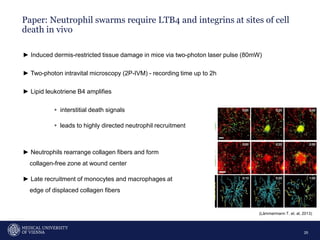
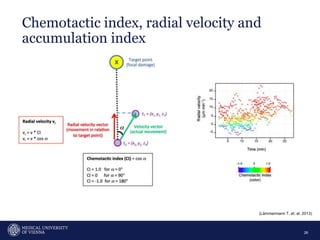
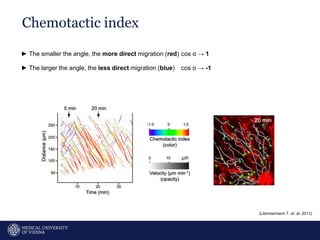
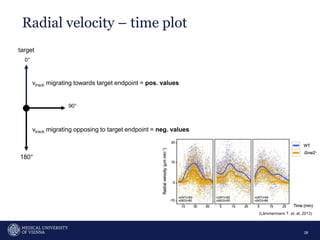
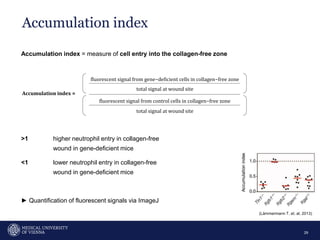
A 32-year-old man presents with headaches and blurred vision for 3 months. MRI shows a space-occupying lesion in the hypophysis. Which of the following tests should be performed in this patient? The document then discusses chemotaxis and various methods to study chemotaxis in vitro and in vivo, including transwell assays, 3D collagen gel assays, and intravital microscopy. Papers are summarized that use these methods to study tumor cell and immune cell chemotaxis and interactions in the tumor microenvironment.