Chem - Practicals.doc
•Download as DOC, PDF•
0 likes•10 views
This document discusses various separation techniques including: 1. Separating insoluble impurities from a soluble substance like removing sand from salt solution. 2. Separating immiscible liquids like water and hexane using a separating funnel. 3. Fractional distillation which separates mixtures of miscible liquids with different boiling points using a fractionating column.
Report
Share
Report
Share
Recommended
Chemistry revision IGCSE 

Atoms are made up of protons, neutrons and electrons. Atoms become ions when they gain or lose electrons. The atomic number is the number of protons and electrons, while the mass number includes protons and neutrons. Isotopes are forms of the same element with different numbers of neutrons. Alkali metals are reactive and found in Group 1. They react vigorously with water. Halogens are in Group 7 and become more reactive going up the group. They react with metals like iron wool. Ionic compounds have high melting points due to strong attractions between oppositely charged ions. Covalent substances share electrons in bonds of varying strengths.
Techniques of separation PPT for class 9 

Filtration can be used to separate an insoluble substance from a soluble substance. It uses a porous barrier such as filter paper to separate solids from liquids, allowing the liquid to pass through while retaining the solid. Chromatography separates components of a mixture based on how strongly they interact with and move across a stationary material like chromatography paper. Distillation separates mixtures based on differences in boiling points, heating the mixture until it vaporizes then cooling the vapors to condense them.
Aqa c3-checklist

The document summarizes key concepts in chemistry including the periodic table, properties of groups in the periodic table, hard and soft water, chemical reactions, and organic compounds. It describes how elements are arranged in the modern periodic table by electronic structure and properties of groups such as metals, nonmetals, and halogens. It also discusses chemical tests and reactions including flame tests, precipitation reactions, acid-base titrations, and the production of ammonia via the Haber process.
MATTER

Here are the types of mixtures for the given examples:
1. Sugar dissolved in water - Solution
2. Mixture of ethyl alcohol and water - Solution
3. Mixture of salt and sand - Heterogeneous mixture (Mechanical mixture)
4. Iron filings, mongo seeds and salt mixture - Heterogeneous mixture (Mechanical mixture)
The first two examples involve dissolving of substances and forming a homogeneous mixture at the molecular level, so they are solutions.
The last two examples involve mechanical mixing of substances without dissolving and remaining as distinguishable parts, so they are heterogeneous mixtures.
All Chem Notes 1 9

This document provides an introduction to chemistry and the scientific method. It discusses chemistry as a science for the 21st century and how it relates to areas like health, energy, materials, food and more. The document then covers topics like the study of chemistry on both the microscopic and macroscopic levels, the scientific method, atomic theory, classification of matter, states of matter, mixtures and pure substances including elements and compounds. It also discusses physical and chemical properties and changes.
G8 Science Q3- Week 3-4- Protons and Atoms.pptx

This document discusses the classification of matter and the properties of solids, liquids, and gases. It begins by defining key terms like elements, compounds, mixtures, homogeneous mixtures, and heterogeneous mixtures. It then discusses the particle nature of matter and how the kinetic energy and attractive forces between particles determines a substance's state. It provides examples of physical and chemical properties and changes. It explains the states of matter and phase changes using diagrams of heating/cooling curves and a phase diagram.
Elements,compounds and mixtures

Elements are pure substances that cannot be broken down further by chemical reactions or processes. They consist of only one type of atom and exist as either individual atoms or molecules made of atoms of the same element. Compounds are pure substances made of two or more elements chemically bonded together in fixed ratios. Compounds have distinct properties and can be broken down into their constituent elements. Mixtures are physical combinations of elements or compounds not chemically bonded. They do not have a fixed composition and their properties depend on the substances that make them up. Mixtures can be separated into their components using physical processes like filtration or evaporation.
chemistry-revision-notes-2012.pdf

This document provides revision notes for the Edexcel IGCSE Chemistry exam. It covers topics like atomic structure, bonding, organic chemistry, calculations, the periodic table, acids and bases. For each topic, it provides definitions, explanations and examples. It also explains techniques for separating mixtures and compounds, including filtration, distillation, chromatography and dissolving. In addition, it discusses the structure of atoms and ions, including electrons, protons and neutrons. It describes the three main types of bonding - ionic, covalent and metallic - and provides examples of how bonding occurs between metals and non-metals to form ions.
Recommended
Chemistry revision IGCSE 

Atoms are made up of protons, neutrons and electrons. Atoms become ions when they gain or lose electrons. The atomic number is the number of protons and electrons, while the mass number includes protons and neutrons. Isotopes are forms of the same element with different numbers of neutrons. Alkali metals are reactive and found in Group 1. They react vigorously with water. Halogens are in Group 7 and become more reactive going up the group. They react with metals like iron wool. Ionic compounds have high melting points due to strong attractions between oppositely charged ions. Covalent substances share electrons in bonds of varying strengths.
Techniques of separation PPT for class 9 

Filtration can be used to separate an insoluble substance from a soluble substance. It uses a porous barrier such as filter paper to separate solids from liquids, allowing the liquid to pass through while retaining the solid. Chromatography separates components of a mixture based on how strongly they interact with and move across a stationary material like chromatography paper. Distillation separates mixtures based on differences in boiling points, heating the mixture until it vaporizes then cooling the vapors to condense them.
Aqa c3-checklist

The document summarizes key concepts in chemistry including the periodic table, properties of groups in the periodic table, hard and soft water, chemical reactions, and organic compounds. It describes how elements are arranged in the modern periodic table by electronic structure and properties of groups such as metals, nonmetals, and halogens. It also discusses chemical tests and reactions including flame tests, precipitation reactions, acid-base titrations, and the production of ammonia via the Haber process.
MATTER

Here are the types of mixtures for the given examples:
1. Sugar dissolved in water - Solution
2. Mixture of ethyl alcohol and water - Solution
3. Mixture of salt and sand - Heterogeneous mixture (Mechanical mixture)
4. Iron filings, mongo seeds and salt mixture - Heterogeneous mixture (Mechanical mixture)
The first two examples involve dissolving of substances and forming a homogeneous mixture at the molecular level, so they are solutions.
The last two examples involve mechanical mixing of substances without dissolving and remaining as distinguishable parts, so they are heterogeneous mixtures.
All Chem Notes 1 9

This document provides an introduction to chemistry and the scientific method. It discusses chemistry as a science for the 21st century and how it relates to areas like health, energy, materials, food and more. The document then covers topics like the study of chemistry on both the microscopic and macroscopic levels, the scientific method, atomic theory, classification of matter, states of matter, mixtures and pure substances including elements and compounds. It also discusses physical and chemical properties and changes.
G8 Science Q3- Week 3-4- Protons and Atoms.pptx

This document discusses the classification of matter and the properties of solids, liquids, and gases. It begins by defining key terms like elements, compounds, mixtures, homogeneous mixtures, and heterogeneous mixtures. It then discusses the particle nature of matter and how the kinetic energy and attractive forces between particles determines a substance's state. It provides examples of physical and chemical properties and changes. It explains the states of matter and phase changes using diagrams of heating/cooling curves and a phase diagram.
Elements,compounds and mixtures

Elements are pure substances that cannot be broken down further by chemical reactions or processes. They consist of only one type of atom and exist as either individual atoms or molecules made of atoms of the same element. Compounds are pure substances made of two or more elements chemically bonded together in fixed ratios. Compounds have distinct properties and can be broken down into their constituent elements. Mixtures are physical combinations of elements or compounds not chemically bonded. They do not have a fixed composition and their properties depend on the substances that make them up. Mixtures can be separated into their components using physical processes like filtration or evaporation.
chemistry-revision-notes-2012.pdf

This document provides revision notes for the Edexcel IGCSE Chemistry exam. It covers topics like atomic structure, bonding, organic chemistry, calculations, the periodic table, acids and bases. For each topic, it provides definitions, explanations and examples. It also explains techniques for separating mixtures and compounds, including filtration, distillation, chromatography and dissolving. In addition, it discusses the structure of atoms and ions, including electrons, protons and neutrons. It describes the three main types of bonding - ionic, covalent and metallic - and provides examples of how bonding occurs between metals and non-metals to form ions.
Classifying Matter

Matter is anything that has mass and takes up space. It is composed of atoms, which contain protons, neutrons, and electrons. Elements are pure substances made of only one type of atom, while compounds are made of two or more elements chemically bonded together. Mixtures contain different substances mixed together but not chemically combined. The three main states of matter are solids, liquids, and gases. Chemical and physical properties can be used to describe and identify matter.
Aqa gcse chemistry c3 revision

The document discusses the history and modern understanding of the periodic table. It covers how elements are arranged based on proton number and how this explains trends in properties within groups. Specific groups like alkali metals, halogens, and transition metals are examined in terms of their structures, properties, and reactions. Common acid-base reactions and quantitative chemical calculations are also summarized.
Ch 17sec3

This document summarizes key concepts about aqueous solutions, including:
- Solutions contain a solvent and one or more solutes
- Ionic compounds generally dissolve well in water due to ion separation through solvation
- "Like dissolves like" - polar substances dissolve in water and nonpolar substances do not
- Electrolytes conduct electricity in solution while nonelectrolytes do not
- Hydrates contain water within their crystalline structure.
Notes main points FROM THE CHAPTER IS MATTER AREOUND US PURE

1. Matter can exist as pure substances, mixtures, or compounds. Pure substances are either elements or compounds that have a uniform composition. Mixtures are combinations of substances that retain their individual chemical identities.
2. Mixtures can be either homogeneous, with a uniform composition, or heterogeneous, with a non-uniform composition. Common homogeneous mixtures include solutions and alloys. Common heterogeneous mixtures include mixtures of sand and sugar.
3. There are various techniques that can be used to separate mixtures into their individual components, including filtration, centrifugation, chromatography, distillation, crystallization, and more. The appropriate separation technique depends on the types of substances involved.
C2 revision powerpoint

The document discusses topics related to chemical reactions and the periodic table. It provides information on:
- Mendeleev's creation of the periodic table and how he arranged elements based on their properties.
- The structure of atoms consisting of protons, neutrons, and electrons located in electron shells around the nucleus.
- The modern periodic table including atomic number and mass number.
- Ionic bonding forming between metals and non-metals through the transfer of electrons. Ionic compounds have high melting/boiling points and conduct electricity when molten or dissolved.
- Covalent bonding forming when atoms share electrons in covalent molecules. Simple covalent substances have low melting/boiling points while giant
presentation of solution of chemistry ppt

This document discusses key concepts in solution chemistry including:
- The definition of a solution as a homogeneous mixture of two or more substances, with the substance present in greater quantity called the solvent and the other(s) called the solute(s).
- Different types of solutions such as saturated, unsaturated, and supersaturated solutions. Supersaturated solutions contain more solute than normally possible.
- Factors that affect solubility including temperature, pressure, and whether the solute and solvent are polar or nonpolar.
- Concentration units used to quantify the amount of solute in a solution, including molarity.
- Colligative properties - properties of solutions that depend only on the number
Chemistry project

This document provides information about solutions and their properties. It discusses several topics:
1. Electrolytes and non-electrolytes - Solutions that conduct electricity, like those containing ions, are electrolytes. Vinegar contains acetic acid which is only partially dissociated.
2. Electrolysis - Passing electricity through a solution can cause a chemical change, in a process called electrolysis. Electrolytic cells use an external energy source to drive nonspontaneous reactions.
3. Colligative properties - Properties like boiling point elevation and freezing point depression depend only on the amount of solute, not its identity. These include vapor pressure lowering, boiling point increase, freezing point depression, and
Chemistry project

This document provides information about solutions and their properties. It discusses several topics:
1. Electrolytes and non-electrolytes - Solutions that conduct electricity, like those containing ions, are electrolytes. Vinegar contains acetic acid which is only partially dissociated.
2. Electrolysis - Passing electricity through a solution can cause a chemical change, in a process called electrolysis. Electrolytic cells use an external energy source to drive nonspontaneous reactions.
3. Colligative properties - Properties like boiling point elevation and freezing point depression depend only on the amount of solute, not its identity. These include vapor pressure lowering, boiling point increase, freezing point depression, and
MixturesandPureSubstances (1).ppt

An atom is the basic unit of an element, just as an element is the basic unit of a compound.
An atom is to an element as a single unit is to the simplest substance. An element consists of multiple identical atoms bonded together.
An atom is to a molecule as a single unit is to multiple units. A molecule is formed when two or more different atoms bond together chemically, whereas an atom is a single unit of an element.
An atom is to a compound molecule as a single unit is to multiple different units. A compound is made of two or more different elements chemically bonded together to form a new substance, with a compound molecule consisting of multiple different bonded atoms.
In summary, atoms are
U13 Everything

1. The document discusses the fundamental properties and states of matter, including solids, liquids, and gases.
2. It describes how matter can change between different states through processes like melting, boiling, condensation, and sublimation.
3. The document also covers topics like particle theory, mixtures, solutions, and separating mixtures using various methods like filtration and distillation.
Chemistry project

This document provides information about solutions and their properties. It discusses several topics:
1. Electrolytes are solutions that conduct electricity due to the presence of ions, while non-electrolytes do not conduct. Vinegar contains acetic acid which is only partially dissociated into ions.
2. Electrolysis is the process of using an electric current to drive nonspontaneous chemical reactions. It involves two electrodes and an electrolyte solution.
3. Colligative properties depend only on the number of solute particles and not their identity. These properties include lowering of vapor pressure, boiling point elevation, freezing point depression, and osmotic pressure.
Elements, compounds, mixtures

This document discusses the key differences between elements, compounds, and mixtures. Elements are basic substances that cannot be broken down further through chemical methods. Compounds are formed when two or more elements chemically combine and have properties different from their components. Mixtures contain two or more substances that can be physically separated. The document then outlines several common techniques for separating mixtures, including filtration, decantation, evaporation, crystallization, centrifugation, distillation, chromatography, and solvent extraction.
Alloys

Alloys are combinations or mixtures of elements.
Metals are alloyed to improve on properties of pure metals such as hardness, strength, corrosion resistance, etc.
Ex.:- Instead of pure aluminum an alloy of aluminum having a combination of Al –Zn –Mg – Cu – Mn(A five-element alloy ) is used to construct the aircraft body.
Alloys may behave differently when combined, some mix easily while others will only be soluble to a limited extent.
TAKS Review Ppt Objective 4

The document discusses various properties of matter and chemical changes. It provides information on:
1) Density being a ratio and not dependent on size. Ice having a lower density than water and therefore floating.
2) Buoyancy causing less dense substances to float in more dense liquids. Boats being made of lower density materials than water.
3) Viscosity being the resistance to flow, with cold syrup having a higher viscosity than warm syrup due to particle interactions.
Introduction

This document discusses the classification and states of matter. It defines substances as samples of matter with uniform physical and chemical properties throughout. Substances can exist in solid, liquid, or gas states depending on the energy and spacing of their particles. Matter is classified as elements, compounds, or mixtures. Elements consist of only one type of atom and cannot be broken down further. Compounds are formed from two or more elements and can be broken down into their component elements. Mixtures contain two or more substances but are not chemically combined. Mixtures can be homogeneous, with uniform composition, or heterogeneous. The document then discusses various states of matter and techniques for separating mixtures.
Chemistry

This document provides a summary of key chemistry concepts covered on the Regents exam, including:
1) Elements cannot be broken down chemically, while mixtures contain two or more physically combined elements or substances.
2) Chemical changes result in new substances with different properties, while physical changes do not alter the identity of the substance.
3) Gas laws relate the pressure, volume, temperature, and amount of gas in chemical reactions and problems.
4) Atoms are made up of protons, neutrons, and electrons. The number of protons determines the element.
5) Chemical bonds, including ionic and covalent bonds, result from the transfer or sharing of electrons between atoms.
Is Matter Around Us Pure ? CLASS - 9

This PowerPoint Presentation will help the students of Class 9 to rejuvenate and to revise what they have learnt so far and to clear their doubts regarding any topic in this Chapter.
O level-chemistry-notes

This document provides information about chemistry concepts including experimental chemistry, atomic structure, and the particulate nature of matter. It discusses measuring volumes of liquids and gases, methods of purification like filtration, crystallization, and distillation. It also describes kinetic particle theory and the states of matter. Atomic structure is explained including protons, neutrons, electrons and isotopes. The document also discusses elements, compounds and mixtures as well as bonding and molecular structure.
Metallurgy -All about it

Metallurgy is the process of extracting metals from ores and purifying them. It involves various physical and chemical steps. Key physical steps include crushing ores, concentrating them using processes like magnetic separation or flotation, and mechanically separating gangue from ores. Chemical steps include roasting or calcination to remove impurities, reduction of metal oxides using coke or other reducing agents, and electrolytic refining to obtain pure metals. The overall metallurgy process allows extraction of metals from ores on a commercial scale.
Properties of Water

Water is made of two hydrogen atoms bonded to one oxygen atom, forming the chemical formula H2O. Water molecules are polar due to the uneven distribution of electrons, giving the oxygen end a partial negative charge and the hydrogen ends partial positive charges. This polarity allows water molecules to form hydrogen bonds with nearby water molecules, giving water its unique properties. These hydrogen bonds allow water to have high surface tension and heat capacity, resist changes in temperature and state, and be an excellent solvent.
Accounting for Restricted Grants When and How To Record Properly

In this webinar, member learned how to stay in compliance with generally accepted accounting principles (GAAP) for restricted grants.
More Related Content
Similar to Chem - Practicals.doc
Classifying Matter

Matter is anything that has mass and takes up space. It is composed of atoms, which contain protons, neutrons, and electrons. Elements are pure substances made of only one type of atom, while compounds are made of two or more elements chemically bonded together. Mixtures contain different substances mixed together but not chemically combined. The three main states of matter are solids, liquids, and gases. Chemical and physical properties can be used to describe and identify matter.
Aqa gcse chemistry c3 revision

The document discusses the history and modern understanding of the periodic table. It covers how elements are arranged based on proton number and how this explains trends in properties within groups. Specific groups like alkali metals, halogens, and transition metals are examined in terms of their structures, properties, and reactions. Common acid-base reactions and quantitative chemical calculations are also summarized.
Ch 17sec3

This document summarizes key concepts about aqueous solutions, including:
- Solutions contain a solvent and one or more solutes
- Ionic compounds generally dissolve well in water due to ion separation through solvation
- "Like dissolves like" - polar substances dissolve in water and nonpolar substances do not
- Electrolytes conduct electricity in solution while nonelectrolytes do not
- Hydrates contain water within their crystalline structure.
Notes main points FROM THE CHAPTER IS MATTER AREOUND US PURE

1. Matter can exist as pure substances, mixtures, or compounds. Pure substances are either elements or compounds that have a uniform composition. Mixtures are combinations of substances that retain their individual chemical identities.
2. Mixtures can be either homogeneous, with a uniform composition, or heterogeneous, with a non-uniform composition. Common homogeneous mixtures include solutions and alloys. Common heterogeneous mixtures include mixtures of sand and sugar.
3. There are various techniques that can be used to separate mixtures into their individual components, including filtration, centrifugation, chromatography, distillation, crystallization, and more. The appropriate separation technique depends on the types of substances involved.
C2 revision powerpoint

The document discusses topics related to chemical reactions and the periodic table. It provides information on:
- Mendeleev's creation of the periodic table and how he arranged elements based on their properties.
- The structure of atoms consisting of protons, neutrons, and electrons located in electron shells around the nucleus.
- The modern periodic table including atomic number and mass number.
- Ionic bonding forming between metals and non-metals through the transfer of electrons. Ionic compounds have high melting/boiling points and conduct electricity when molten or dissolved.
- Covalent bonding forming when atoms share electrons in covalent molecules. Simple covalent substances have low melting/boiling points while giant
presentation of solution of chemistry ppt

This document discusses key concepts in solution chemistry including:
- The definition of a solution as a homogeneous mixture of two or more substances, with the substance present in greater quantity called the solvent and the other(s) called the solute(s).
- Different types of solutions such as saturated, unsaturated, and supersaturated solutions. Supersaturated solutions contain more solute than normally possible.
- Factors that affect solubility including temperature, pressure, and whether the solute and solvent are polar or nonpolar.
- Concentration units used to quantify the amount of solute in a solution, including molarity.
- Colligative properties - properties of solutions that depend only on the number
Chemistry project

This document provides information about solutions and their properties. It discusses several topics:
1. Electrolytes and non-electrolytes - Solutions that conduct electricity, like those containing ions, are electrolytes. Vinegar contains acetic acid which is only partially dissociated.
2. Electrolysis - Passing electricity through a solution can cause a chemical change, in a process called electrolysis. Electrolytic cells use an external energy source to drive nonspontaneous reactions.
3. Colligative properties - Properties like boiling point elevation and freezing point depression depend only on the amount of solute, not its identity. These include vapor pressure lowering, boiling point increase, freezing point depression, and
Chemistry project

This document provides information about solutions and their properties. It discusses several topics:
1. Electrolytes and non-electrolytes - Solutions that conduct electricity, like those containing ions, are electrolytes. Vinegar contains acetic acid which is only partially dissociated.
2. Electrolysis - Passing electricity through a solution can cause a chemical change, in a process called electrolysis. Electrolytic cells use an external energy source to drive nonspontaneous reactions.
3. Colligative properties - Properties like boiling point elevation and freezing point depression depend only on the amount of solute, not its identity. These include vapor pressure lowering, boiling point increase, freezing point depression, and
MixturesandPureSubstances (1).ppt

An atom is the basic unit of an element, just as an element is the basic unit of a compound.
An atom is to an element as a single unit is to the simplest substance. An element consists of multiple identical atoms bonded together.
An atom is to a molecule as a single unit is to multiple units. A molecule is formed when two or more different atoms bond together chemically, whereas an atom is a single unit of an element.
An atom is to a compound molecule as a single unit is to multiple different units. A compound is made of two or more different elements chemically bonded together to form a new substance, with a compound molecule consisting of multiple different bonded atoms.
In summary, atoms are
U13 Everything

1. The document discusses the fundamental properties and states of matter, including solids, liquids, and gases.
2. It describes how matter can change between different states through processes like melting, boiling, condensation, and sublimation.
3. The document also covers topics like particle theory, mixtures, solutions, and separating mixtures using various methods like filtration and distillation.
Chemistry project

This document provides information about solutions and their properties. It discusses several topics:
1. Electrolytes are solutions that conduct electricity due to the presence of ions, while non-electrolytes do not conduct. Vinegar contains acetic acid which is only partially dissociated into ions.
2. Electrolysis is the process of using an electric current to drive nonspontaneous chemical reactions. It involves two electrodes and an electrolyte solution.
3. Colligative properties depend only on the number of solute particles and not their identity. These properties include lowering of vapor pressure, boiling point elevation, freezing point depression, and osmotic pressure.
Elements, compounds, mixtures

This document discusses the key differences between elements, compounds, and mixtures. Elements are basic substances that cannot be broken down further through chemical methods. Compounds are formed when two or more elements chemically combine and have properties different from their components. Mixtures contain two or more substances that can be physically separated. The document then outlines several common techniques for separating mixtures, including filtration, decantation, evaporation, crystallization, centrifugation, distillation, chromatography, and solvent extraction.
Alloys

Alloys are combinations or mixtures of elements.
Metals are alloyed to improve on properties of pure metals such as hardness, strength, corrosion resistance, etc.
Ex.:- Instead of pure aluminum an alloy of aluminum having a combination of Al –Zn –Mg – Cu – Mn(A five-element alloy ) is used to construct the aircraft body.
Alloys may behave differently when combined, some mix easily while others will only be soluble to a limited extent.
TAKS Review Ppt Objective 4

The document discusses various properties of matter and chemical changes. It provides information on:
1) Density being a ratio and not dependent on size. Ice having a lower density than water and therefore floating.
2) Buoyancy causing less dense substances to float in more dense liquids. Boats being made of lower density materials than water.
3) Viscosity being the resistance to flow, with cold syrup having a higher viscosity than warm syrup due to particle interactions.
Introduction

This document discusses the classification and states of matter. It defines substances as samples of matter with uniform physical and chemical properties throughout. Substances can exist in solid, liquid, or gas states depending on the energy and spacing of their particles. Matter is classified as elements, compounds, or mixtures. Elements consist of only one type of atom and cannot be broken down further. Compounds are formed from two or more elements and can be broken down into their component elements. Mixtures contain two or more substances but are not chemically combined. Mixtures can be homogeneous, with uniform composition, or heterogeneous. The document then discusses various states of matter and techniques for separating mixtures.
Chemistry

This document provides a summary of key chemistry concepts covered on the Regents exam, including:
1) Elements cannot be broken down chemically, while mixtures contain two or more physically combined elements or substances.
2) Chemical changes result in new substances with different properties, while physical changes do not alter the identity of the substance.
3) Gas laws relate the pressure, volume, temperature, and amount of gas in chemical reactions and problems.
4) Atoms are made up of protons, neutrons, and electrons. The number of protons determines the element.
5) Chemical bonds, including ionic and covalent bonds, result from the transfer or sharing of electrons between atoms.
Is Matter Around Us Pure ? CLASS - 9

This PowerPoint Presentation will help the students of Class 9 to rejuvenate and to revise what they have learnt so far and to clear their doubts regarding any topic in this Chapter.
O level-chemistry-notes

This document provides information about chemistry concepts including experimental chemistry, atomic structure, and the particulate nature of matter. It discusses measuring volumes of liquids and gases, methods of purification like filtration, crystallization, and distillation. It also describes kinetic particle theory and the states of matter. Atomic structure is explained including protons, neutrons, electrons and isotopes. The document also discusses elements, compounds and mixtures as well as bonding and molecular structure.
Metallurgy -All about it

Metallurgy is the process of extracting metals from ores and purifying them. It involves various physical and chemical steps. Key physical steps include crushing ores, concentrating them using processes like magnetic separation or flotation, and mechanically separating gangue from ores. Chemical steps include roasting or calcination to remove impurities, reduction of metal oxides using coke or other reducing agents, and electrolytic refining to obtain pure metals. The overall metallurgy process allows extraction of metals from ores on a commercial scale.
Properties of Water

Water is made of two hydrogen atoms bonded to one oxygen atom, forming the chemical formula H2O. Water molecules are polar due to the uneven distribution of electrons, giving the oxygen end a partial negative charge and the hydrogen ends partial positive charges. This polarity allows water molecules to form hydrogen bonds with nearby water molecules, giving water its unique properties. These hydrogen bonds allow water to have high surface tension and heat capacity, resist changes in temperature and state, and be an excellent solvent.
Similar to Chem - Practicals.doc (20)
Notes main points FROM THE CHAPTER IS MATTER AREOUND US PURE

Notes main points FROM THE CHAPTER IS MATTER AREOUND US PURE
Recently uploaded
Accounting for Restricted Grants When and How To Record Properly

In this webinar, member learned how to stay in compliance with generally accepted accounting principles (GAAP) for restricted grants.
How to Manage Reception Report in Odoo 17

A business may deal with both sales and purchases occasionally. They buy things from vendors and then sell them to their customers. Such dealings can be confusing at times. Because multiple clients may inquire about the same product at the same time, after purchasing those products, customers must be assigned to them. Odoo has a tool called Reception Report that can be used to complete this assignment. By enabling this, a reception report comes automatically after confirming a receipt, from which we can assign products to orders.
CHUYÊN ĐỀ ÔN TẬP VÀ PHÁT TRIỂN CÂU HỎI TRONG ĐỀ MINH HỌA THI TỐT NGHIỆP THPT ...

CHUYÊN ĐỀ ÔN TẬP VÀ PHÁT TRIỂN CÂU HỎI TRONG ĐỀ MINH HỌA THI TỐT NGHIỆP THPT ...Nguyen Thanh Tu Collection
https://app.box.com/s/qspvswamcohjtbvbbhjad04lg65waylfBossa N’ Roll Records by Ismael Vazquez.

Bossa N Roll Records presentation by Izzy Vazquez for Music Retail and Distribution class at Full Sail University
How to Fix [Errno 98] address already in use![How to Fix [Errno 98] address already in use](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![How to Fix [Errno 98] address already in use](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
This slide will represent the cause of the error “[Errno 98] address already in use” and the troubleshooting steps to resolve this error in Odoo.
How to Download & Install Module From the Odoo App Store in Odoo 17

Custom modules offer the flexibility to extend Odoo's capabilities, address unique requirements, and optimize workflows to align seamlessly with your organization's processes. By leveraging custom modules, businesses can unlock greater efficiency, productivity, and innovation, empowering them to stay competitive in today's dynamic market landscape. In this tutorial, we'll guide you step by step on how to easily download and install modules from the Odoo App Store.
Level 3 NCEA - NZ: A Nation In the Making 1872 - 1900 SML.ppt

The History of NZ 1870-1900.
Making of a Nation.
From the NZ Wars to Liberals,
Richard Seddon, George Grey,
Social Laboratory, New Zealand,
Confiscations, Kotahitanga, Kingitanga, Parliament, Suffrage, Repudiation, Economic Change, Agriculture, Gold Mining, Timber, Flax, Sheep, Dairying,
BPSC-105 important questions for june term end exam

BPSC-105 important questions for june term end exam
A Visual Guide to 1 Samuel | A Tale of Two Hearts

These slides walk through the story of 1 Samuel. Samuel is the last judge of Israel. The people reject God and want a king. Saul is anointed as the first king, but he is not a good king. David, the shepherd boy is anointed and Saul is envious of him. David shows honor while Saul continues to self destruct.
KHUSWANT SINGH.pptx ALL YOU NEED TO KNOW ABOUT KHUSHWANT SINGH

INDIA`S OWN LITERARY GENIUS MR.KHUSHWANT SINGH WAS TRULY A VERY BRAVE SOUL AND WAS AWARDED WITH THE MAGIC OF WORDS BY GOD.
مصحف القراءات العشر أعد أحرف الخلاف سمير بسيوني.pdf

مصحف أحرف الخلاف للقراء العشرةأعد أحرف الخلاف بالتلوين وصلا سمير بسيوني غفر الله له
Recently uploaded (20)
Accounting for Restricted Grants When and How To Record Properly

Accounting for Restricted Grants When and How To Record Properly
CHUYÊN ĐỀ ÔN TẬP VÀ PHÁT TRIỂN CÂU HỎI TRONG ĐỀ MINH HỌA THI TỐT NGHIỆP THPT ...

CHUYÊN ĐỀ ÔN TẬP VÀ PHÁT TRIỂN CÂU HỎI TRONG ĐỀ MINH HỌA THI TỐT NGHIỆP THPT ...
How to Download & Install Module From the Odoo App Store in Odoo 17

How to Download & Install Module From the Odoo App Store in Odoo 17
Level 3 NCEA - NZ: A Nation In the Making 1872 - 1900 SML.ppt

Level 3 NCEA - NZ: A Nation In the Making 1872 - 1900 SML.ppt
BPSC-105 important questions for june term end exam

BPSC-105 important questions for june term end exam
REASIGNACION 2024 UGEL CHUPACA 2024 UGEL CHUPACA.pdf

REASIGNACION 2024 UGEL CHUPACA 2024 UGEL CHUPACA.pdf
Juneteenth Freedom Day 2024 David Douglas School District

Juneteenth Freedom Day 2024 David Douglas School District
KHUSWANT SINGH.pptx ALL YOU NEED TO KNOW ABOUT KHUSHWANT SINGH

KHUSWANT SINGH.pptx ALL YOU NEED TO KNOW ABOUT KHUSHWANT SINGH
مصحف القراءات العشر أعد أحرف الخلاف سمير بسيوني.pdf

مصحف القراءات العشر أعد أحرف الخلاف سمير بسيوني.pdf
Chem - Practicals.doc
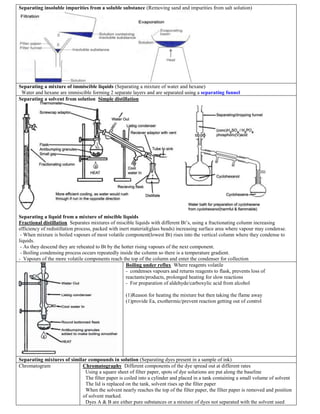
- 1. Separating insoluble impurities from a soluble substance (Removing sand and impurities from salt solution) Separating a mixture of immiscible liquids (Separating a mixture of water and hexane) Water and hexane are immiscible forming 2 separate layers and are separated using a separating funnel Separating a solvent from solution Simple distillation Separating a liquid from a mixture of miscible liquids Fractional distillation Separates mixtures of miscible liquids with different Bt’s, using a fractionating column increasing efficiency of redistillation process, packed with inert material(glass beads) increasing surface area where vapour may condense. - When mixture is boiled vapours of most volatile component(lowest Bt) rises into the vertical column where they condense to liquids. - As they descend they are reheated to Bt by the hotter rising vapours of the next component. - Boiling condensing process occurs repeatedly inside the column so there is a temperature gradient. - Vapours of the more volatile components reach the top of the column and enter the condenser for collection Boiling under reflux Where reagents volatile - condenses vapours and returns reagents to flask, prevents loss of reactants/products, prolonged heating for slow reactions - For preparation of aldehyde/carboxylic acid from alcohol (1)Reason for heating the mixture but then taking the flame away (1)provide Ea, exothermic/prevent reaction getting out of control Separating mixtures of similar compounds in solution (Separating dyes present in a sample of ink) Chromatogram Chromatography Different components of the dye spread out at different rates Using a square sheet of filter paper, spots of dye solutions are put along the baseline The filter paper is coiled into a cylinder and placed in a tank containing a small volume of solvent The lid is replaced on the tank, solvent rises up the filter paper When the solvent nearly reaches the top of the filter paper, the filter paper is removed and position of solvent marked. Dyes A & B are either pure substances or a mixture of dyes not separated with the solvent used
- 2. Dye C is composed of A & B as the spots correspond Colourless substances can be separated and seen by spraying/dipping the filter paper into a locating agent which colours the spots produced Separating a solid which sublimes, from a solid which doesn’t sublime Given a mixture of Ammonium chloride(sublimes) and sodium chloride(doesn’t sublime) Heat the mixture. Ammonium chloride turns directly to vapour but the sodium chloride remains unchanged When the vapour is cooled solid ammonium chloride collects free from sodium chloride A pure substance has a definite Mt, presence of impurities causes the substance to melt over a range of temperatures Best method of separation of (1) Oil and water (2) Alcohol and water (3) Nitrogen from liquid air (1) Separating funnel(2) Fractional distillation (3) Fractional distillation Mixture Compound - Proportions of the different elements can be varied - Properties are those of the elements making it up - Elements can be separated by simple methods - No energy gained or lost when the mixture is made - Different elements have to be present in fixed proportions - Properties different from properties of elements making it up - Difficult to separate into the elements which make it up - Energy usually given out/taken in when compound is formed Sub-atomic particles Protons, neutrons and electrons which makes up the atom Particle An atom, molecule, ion, electron or any identifiable particle RTP Room temperature and pressure Electron A negatively charged particle, with negligible mass occupying the outer regions of all atoms Immiscible Unable to mix, dissolve in each other, to form a homogenous mixture Miscible Soluble in each other (aq) Substance dissolved in water to form an aqueous solution State symbols Physical state of the reactants at RT Aqueous(aq) Solvent Substance in which other substances are dissolved Solute Substance dissolved in another substance(solvent)to form a solution Chemical species Collection of particles Distilled water Water that has been purified by distillation Ion When number of protons and electrons are different Atom The smallest part of an element that can exist on its own Molecule 2 or more atoms bonded together Element A pure substance which can’t be split up by chemical reaction Compound Combination of elements in fixed proportions via synthesis In formation of a compound from ions the charges balance out Physical properties: Mt, Bt, hardness • Compounds ending in –ate –ite contain oxygen, greater proportion of oxygen in the compound ending in –ate Sodium sulphate Na2SO4 Sodium sulphite Na2SO3 • Compounds with prefix per– contain extra oxygen Sodium oxide Na2O Sodium peroxide Na2O2 • Compounds with prefix thio– contain a sulphur atom in place of an oxygen atom Sodium sulphate Na2SO4 Sodium thiosulphate Na2S2O3 Metalloid Element which has properties between metals and nonmetals - Ions in an ionic compound are tightly held together in a regular lattice, lattice energy is required to break it up and melt the substance A metal high in the reactivity series has stable ores and the metal can be obtained only by electrolysis A metal middle in the reactivity series doesn’t form stable ores and can be extracted by reduction reactions (often with carbon) A metal low in the reactivity series, if present in unstable ores can be extracted by heating Decomposition Splitting up of a compound (Thermal decomposition - decomposition of a compound by heating) Combustion is the reaction of a substance with oxygen, total mass of products is greater than the mass of the substance burned, difference being the mass of oxygen combined Sublimation of an element/compound is a transition from solid to gas with no intermediate stage When a change of state takes place the temperature remains constant despite a continuing supply of energy. Latent heat is the energy which is not being used to raise the temperature and supplies particles with the extra energy they require as the state changes(given out when the reverse changes take place) Fe(s) + CuSO4(aq) FeSO4(aq) + Cu(s) Blue solution turns colourless and brown copper is deposited A displacement reaction where a more reactive metal replaces a less reactive metal in a compound Electrolysis of HCl(aq): 2HCl(aq) Cl2(g) + H2(g) Cathode: 2H+ (aq) + 2e– H2(g) Anode: 2Cl – (aq)Cl2(g)+2e– Heat Ammonium chloride NH4Cl(s) NH3(g) + HCl(g)
- 3. Cool A stopper from a bottle of (conc)NH3(aq) held near a stopper from a bottle of (conc)HCl acid gives a dense white smoke of NH4Cl