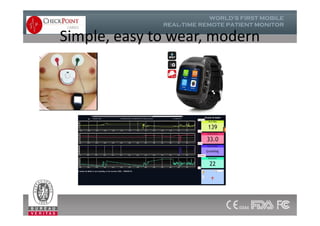
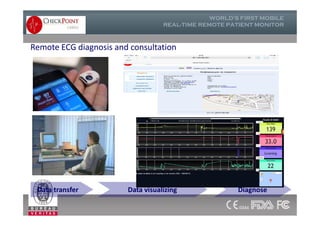
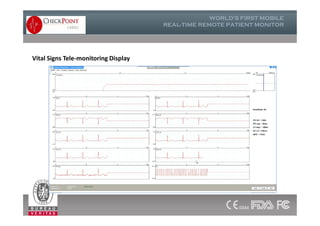
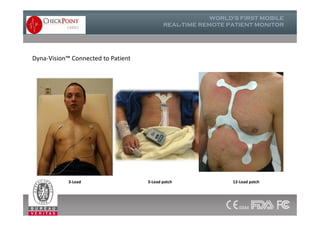
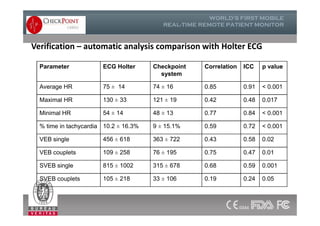
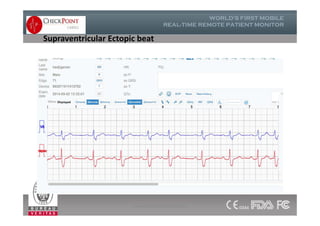
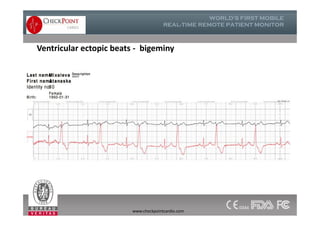
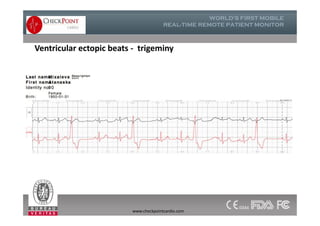
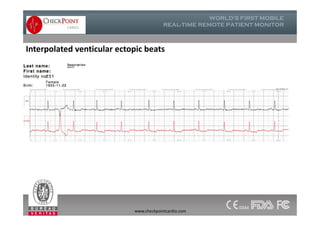
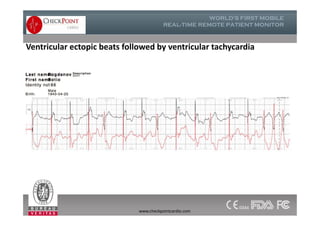
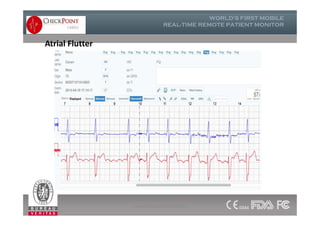
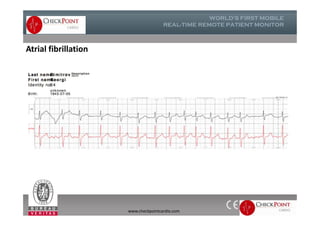
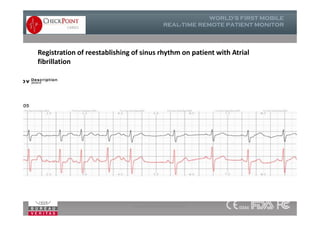
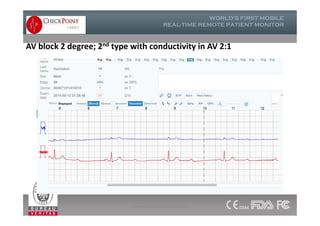
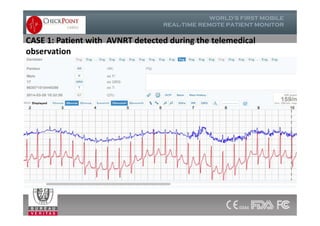
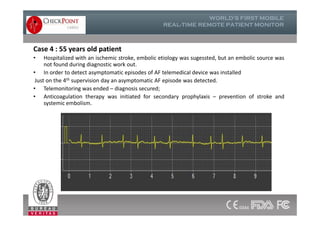
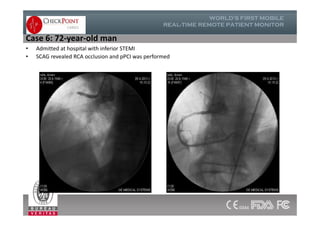
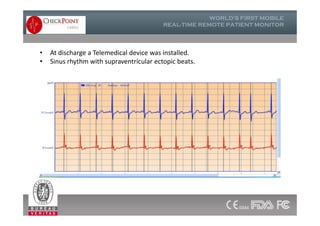
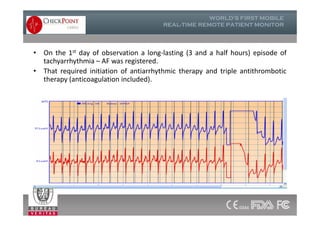
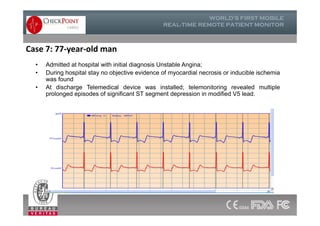
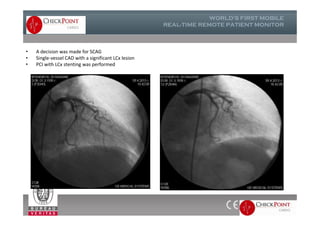
The document discusses Check Point, a company that provides telemonitoring platforms and solutions. It offers two main platforms - one involving a partnership with Vital Connect that allows for ECG, temperature, and other vital sign monitoring using wearable devices. The other involves a partnership with Techmedic International and provides more advanced real-time monitoring and analysis of vital signs like ECG, oxygen levels, and heart rate. Check Point also operates a telemedicine center that provides 24/7 monitoring of patient data and emergency response services for up to 15,000 patients simultaneously.