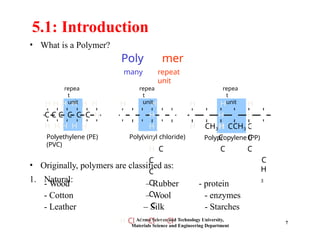
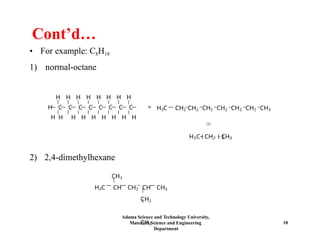
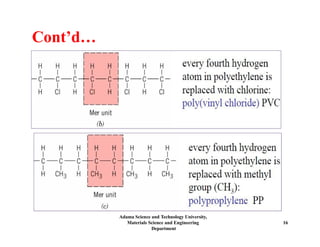
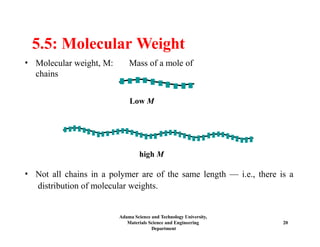
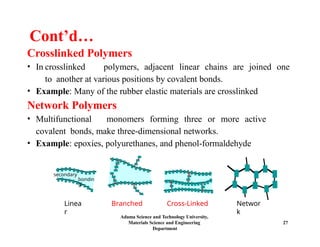
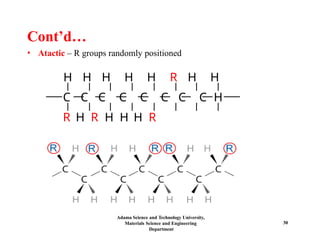
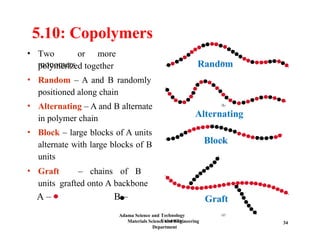
Chapter 5 discusses polymer structure and characteristics, focusing on molecular composition, molecular weight, and configurations of various polymer types. It emphasizes the importance of polymer crystallinity and the distinctions between thermoplastic and thermosetting polymers, detailing their properties and behaviors. Additionally, the chapter outlines learning objectives related to polymer chemistry, structures, and defects within polymeric materials.