This document provides an overview of fundamental properties of water, including:
- Water can exist in three phases (solid, liquid, gas) depending on temperature and pressure, and requires latent heat to change phases.
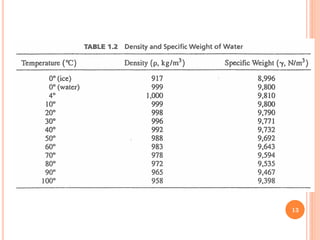
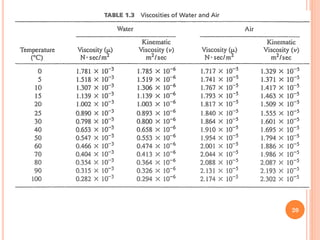
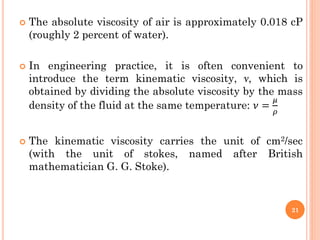
- Water has a maximum density of 1 g/cm3 at 4°C and its density and viscosity depend on temperature and pressure.
- Atmospheric pressure at sea level is approximately 1 bar and exerts pressure on any surface in contact with air, including water surfaces.
- The document defines key terms including density, specific weight, viscosity, vapor pressure, and cavitation.