This document provides an overview of Cancer Genetics, Inc.'s presentation at the RedChip Global Conference on October 15, 2014. The presentation discusses CGI's business focus on developing genomic tests to assess and personalize cancer treatment. It highlights six proprietary diagnostic products that have been commercially launched, as well as CGI's product pipeline. The presentation also summarizes CGI's partnerships with academic and research institutions and its global operations across multiple locations.








![Product Pipeline
11
Company & Portfolio Update | 2014 Cancer Genetics, Inc. | www.cancergenetics.com
Research &
Discovery
Clinical
Development
Commercial
Development
Launch &
Market Entry
Indication
Development Stage
Hematologic Cancers
Multiple Myeloma
Comprehensive Myeloid Panel
[NGS-based]
Comprehensive CLL Panel
[NGS-based]
UroGenital Cancers
Bladder Cancer
Comprehensive Renal Panel
[NGS-based]
Cervical Cancer
HPV-Associated Cancers
(FHACT®)
Head & Neck Cancer](https://image.slidesharecdn.com/cgixpresentation-redchip-141015065244-conversion-gate01/85/Cgix-presentation-red-chip-11-320.jpg)

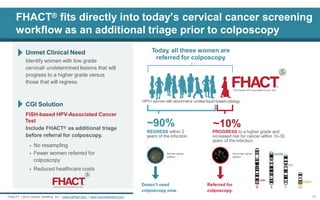
![FHACT® can aid in identifying women with markers of high grade lesions (cervical cancer)
FISH-based HPV-Associated Cancer Test FHACT® assesses non-random genomic alterations associated with progression of lesion.
Gain of 3q26 (TERC) has been detected with increasing frequency in cervical lesions with increasing severity and ultimately is observed in about 75% of cervical cancers.[1-2]
Gains of 5p15, 20q13 & chromosome 7 share a similar pattern of appearance in precancerous cytology specimens by FISH (40- 45% for 5p15 and 20q13, and 15% for chromosome 7).[3-4]
Performed on remnant liquid based cytology (LBC). FHACT® presents the highest sensitivity on the market (4 loci). Gain at any of the FHACT® loci is detected in up to 89.5% of all cervical cancers.[5]
1.Heselmeyer-Haddad K, et al. (2005). Am. J. Pathol. , 166, 1229-1238
2.Seppo A., et al. (2009) Gynecol Oncol, 114, 80-83
3.Scotto, L., et al. (2008). Mol Cancer, 7, 58. 4. Luhn P, et al. (2013). Gynecol Oncol, 130, 595-600. 5. The Cancer Genome Atlas (TCGA) (http:/cancergenome.nih.gov)
chromosome 3
chromosome 7
chromosome 5
chr 20
14
FHACT® Loci:
3q26 gain (red)
5p15 gain (green)
Cen7 (aqua)
20q13 gain (gold)
FHACT® | 2014 Cancer Genetics, Inc. | www.cgifhact.com | www.cancergenetics.com](https://image.slidesharecdn.com/cgixpresentation-redchip-141015065244-conversion-gate01/85/Cgix-presentation-red-chip-14-320.jpg)
![Renal Portfolio | 2014 Cancer Genetics, Inc. | www.cancergenetics.com
15,720 new cases 4,600 deaths
Estimated for 2014 [ACS]
Unmet Needs:
Risk stratification to identify patients most likely to have aggressive disease.
Therapy selection
Chronic Lymphocytic Leukemia (CLL)
Median age at diagnosis is 65 to 68 years
Overall median survival is 9 years
Approximately 10% of all adult hematologic malignancies (40% of leukemias in individuals over 65 years of age)
Two roughly equal clinical subtypes: indolent & aggressive
15
Hematology Portfolio | 2014 Cancer Genetics, Inc. | www.cancergenetics.com
7-8x That Are Living With CLL](https://image.slidesharecdn.com/cgixpresentation-redchip-141015065244-conversion-gate01/85/Cgix-presentation-red-chip-15-320.jpg)
![Independent Validation Datasets: DFCI (124), HUMC (65)
Time to First Treatment (TTFT)
Time (months)
Time (months)
Proportion Surviving
Proportion Treatment- Free
– GOOD (n=74)
– INTERMED (n=107)
– POOR (n=47)
– GOOD (n=74)
– INTERMED (n=107)
– POOR (n=47)
P = 0.090
P = 0.001
P < 0.001
P = 0.010
Overall Survival (OS)
Leukemia & Lymphoma – Houldsworth, et. al Sept. 18, 2013
Time (months)
TTFT: DFCI
Proportion Treatment-Free
P<0.001
Good (n=63) Intermediate (n=47) Poor (n=14)
Time (months)
OS: DFCI
Proportion Surviving
P=0.522
Good (n=63) Intermediate (n=47) Poor (n=14)
Time (months)
TTFT: HUMC
Proportion Treatment-Free
P=0.039
Good (n=13) Intermediate (n=34) Poor (n=18)
Time (months)
OS: HUMC
Proportion Surviving
P=0.044
Good (n=13) Intermediate (n=34) Poor (n=18)
[Jennifer Brown]
[Anthony Mato]
Discovery: 288 specimens
Validation: 124, 65 specimens
17
Hematology Portfolio | 2014 Cancer Genetics, Inc. | www.cancergenetics.com](https://image.slidesharecdn.com/cgixpresentation-redchip-141015065244-conversion-gate01/85/Cgix-presentation-red-chip-17-320.jpg)
![Significance of Current Prognostication Markers in CLL in the B-Cell Receptor Signaling Pathway Inhibitor Era
B-Cell Receptor Signaling Pathway Inhibitors
[Kanti Rai, Nicholas Chiorazzi, Jacqueline Barrientos]
Zydelig [Idelalisib (PI3K-delta)]
FDA Approved For CLL in the relapse setting when considering Rituximab alone
Collaborative project in progress at CGI
Inisights will be integrated into our Complete CLL
IMBRUVICA® [Ibrutinib (BTK)]
FDA Approved for CLL with 17p loss and in the relapse setting where two prior therapies have failed
Genomic alterations associated with resistance
Acquired Mutations during therapy in BTK Gene (C481S), PLCG2 (R665W)
Deletion of 8p
Gain of 3q
18
Hematology Portfolio | 2014 Cancer Genetics, Inc. | www.cancergenetics.com](https://image.slidesharecdn.com/cgixpresentation-redchip-141015065244-conversion-gate01/85/Cgix-presentation-red-chip-18-320.jpg)





![Experienced and Focused Management and Boards
28
Company & Portfolio Update | 2014 Cancer Genetics, Inc. | www.cancergenetics.com
Scientific Advisory Board
Andrea Califano, Ph.D.
Chairman of the Columbia Initiative for Systems Biology
Associate Director for Bioinformatics, Herbert Irving Comprehensive Cancer Ctr
Timothy A. Chan, M.D., Ph.D.
Principal Investigator, Human Oncology and Pathogenesis Program at Memorial Sloan- Kettering Cancer Center
Riccardo Dalla-Favera, M.D.
Director, Institute for Cancer Genetics at Columbia University
Vundavalli V. Murty, Ph.D.
Director, Cancer Cytogenetic Laboratory and Molecular Pathology at Columbia University
Hans-Guido Wendel, M.D.
Principal Investigator, Cancer Genetics Laboratory at Memorial Sloan-Kettering Cancer Center
Howard McLeod, PharmD
Medical Director, DeBartolo Family Personalized Medicine Institute, Moffitt Cancer Center
Andrew D. Zelenetz, M.D., Ph.D.
Chief of Lymphoma Service and Head of Molecular Hemo-Oncology Laboratory, Department of Medicine at MSKCC
Raju Chaganti, Ph.D., FACMG Founder
•35+ years in cancer research; 38 at MSKCC
•Major discoveries in cancer genomics
•Published 350+ articles, 4 patents
Panna Sharma President & CEO
•15+ years as advisor to global life science & healthcare cos.
•Founded TSG Partners
•Chief Strategy Officer, iXL (IIXL)
Edward J. Sitar Chief Financial Officer & Treasurer
•30+ yrs in finance & deal making in the healthcare industry
•Healthagen, ActiveHealth Management, Cadent Holding, MIM Corporation (Bioscrip), Vital Signs, Zenith/Goldline Pharmaceutical, Coopers & Lybrand
Jane Houldsworth, Ph.D. Vice President of R&D
•25+ years in translational oncology research
•Published 50+ articles, 4 patents
•NIH grantee
John Pappajohn [Chairman of the Board]
|
Keith Brownlie, CPA
|
Edmund Cannon
|
Raju Chaganti, Ph.D.
Michael J. Welsh, M.D.
|
Franklyn Prendergast, M.D., Ph.D.
|
Paul Rothman, M.D.
|
Panna Sharma
Board of Directors
Officers & Management Team](https://image.slidesharecdn.com/cgixpresentation-redchip-141015065244-conversion-gate01/85/Cgix-presentation-red-chip-28-320.jpg)
