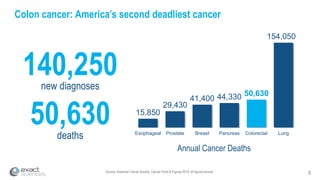
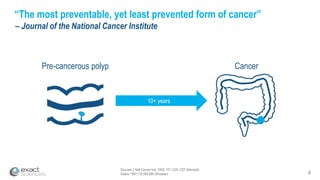
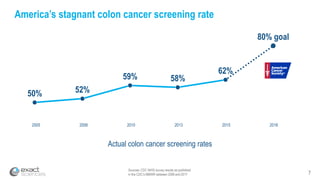
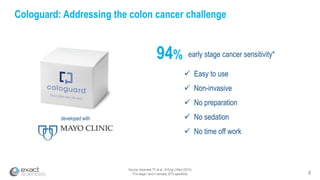
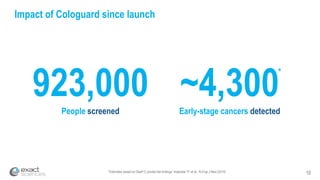
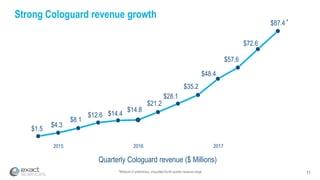
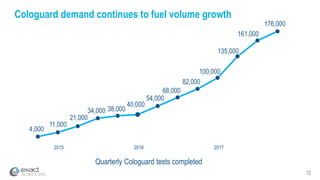
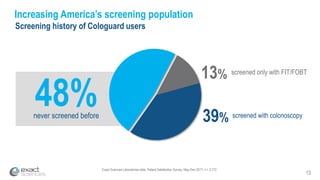
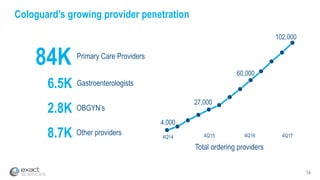
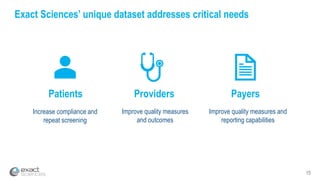
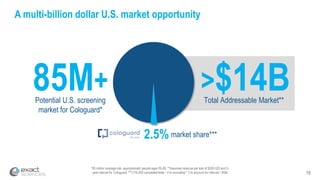
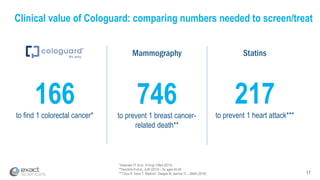
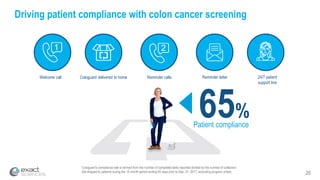
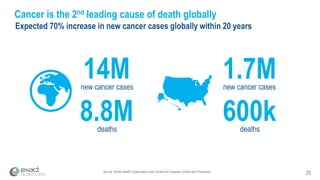
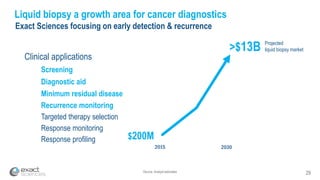
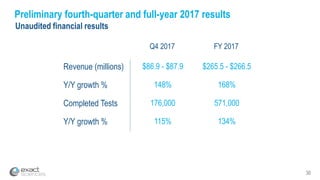
Kevin Conroy, CEO of Exact Sciences, presented at the 36th Annual J.P. Morgan Healthcare Conference. He discussed Exact Sciences' vision of helping win the war on cancer through early detection using Cologuard. Cologuard demand continues to grow, with over 900,000 people screened and 4,300 early-stage cancers detected since launch. Exact Sciences reported preliminary unaudited Q4 2017 revenue of $86.9-$87.9 million and full year revenue of $265.5-$266.5 million, representing strong year-over-year growth. Exact Sciences is also investing in expanding infrastructure, marketing, and its product pipeline to pursue a multi-billion dollar market opportunity in col