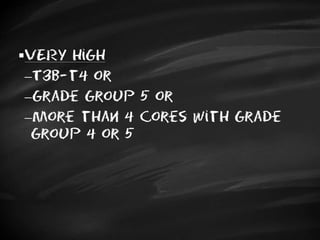This document discusses prostate cancer, including:
- It is the most common cancer in men in North America and the second leading cause of cancer death in men.
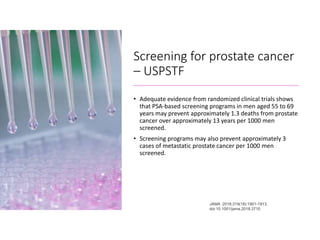
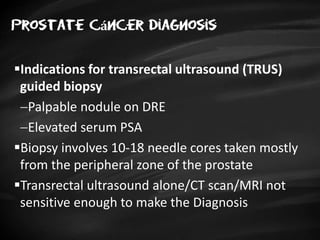
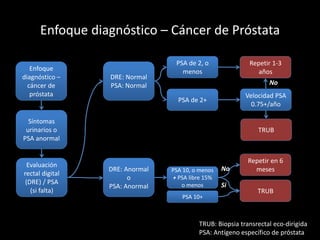
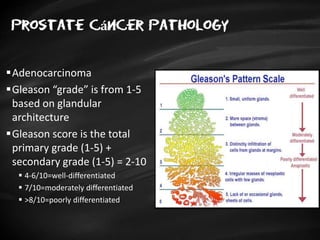
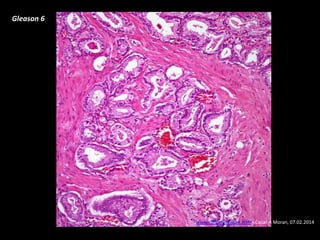
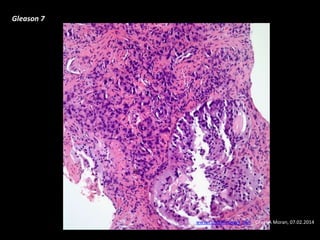
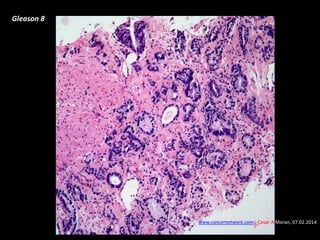
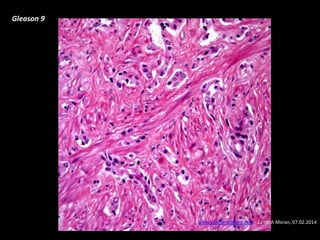
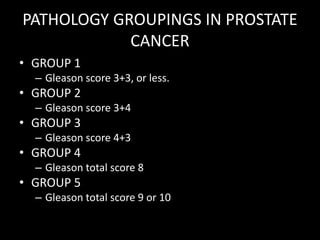
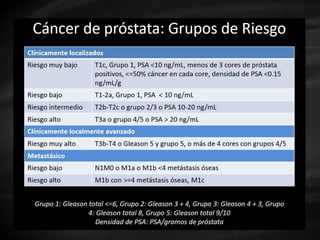
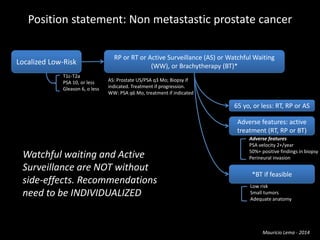
- Risk factors include advancing age, family history, and African ancestry. Screening includes a PSA test and digital rectal exam. Biopsy is used for diagnosis.
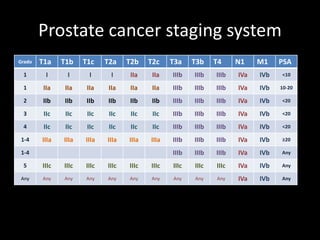
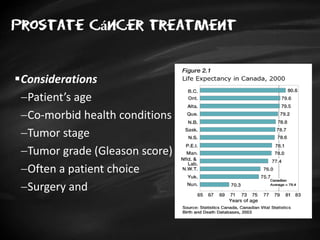
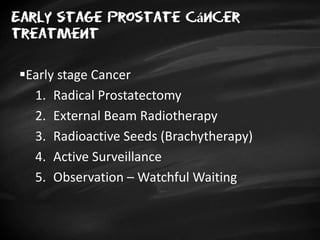
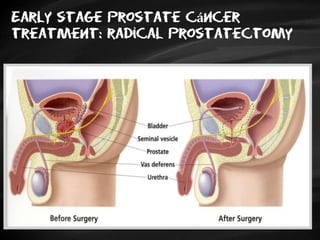
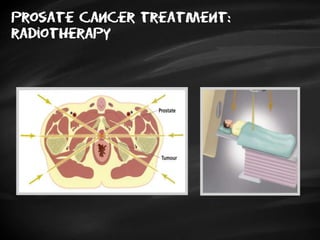
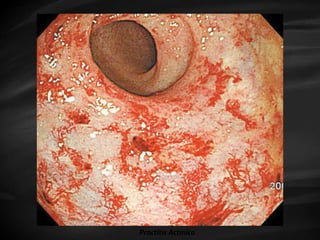
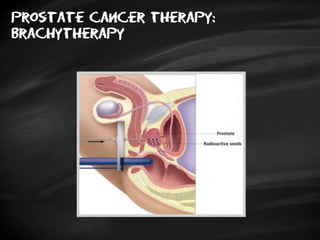
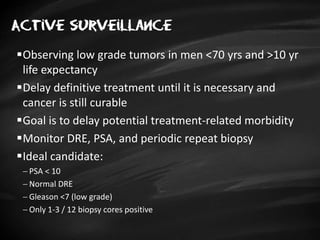
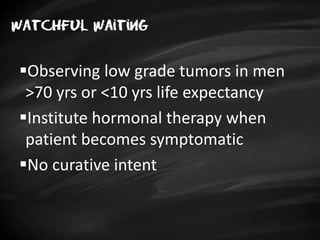
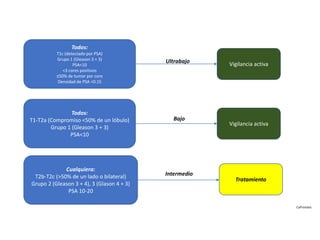
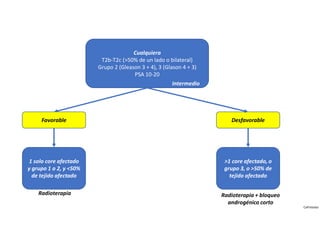
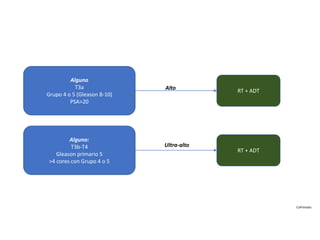
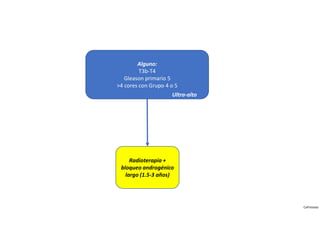
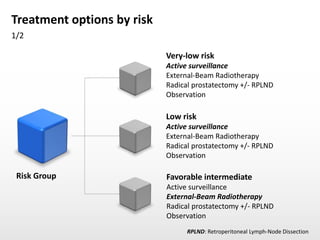
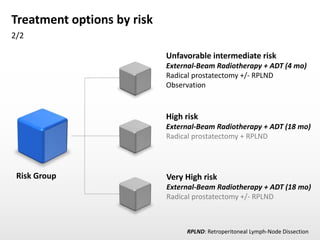
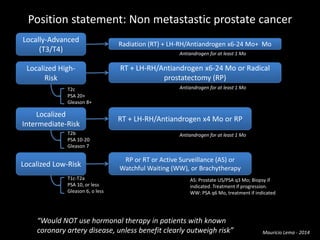
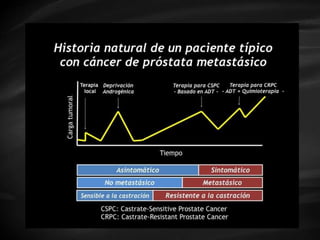
- Treatment options depend on tumor stage and grade. Early stage options include radical prostatectomy, radiotherapy, brachytherapy, active surveillance, or observation. Later stage options involve more aggressive treatments. Complications can include incontinence and erectile dysfunction.