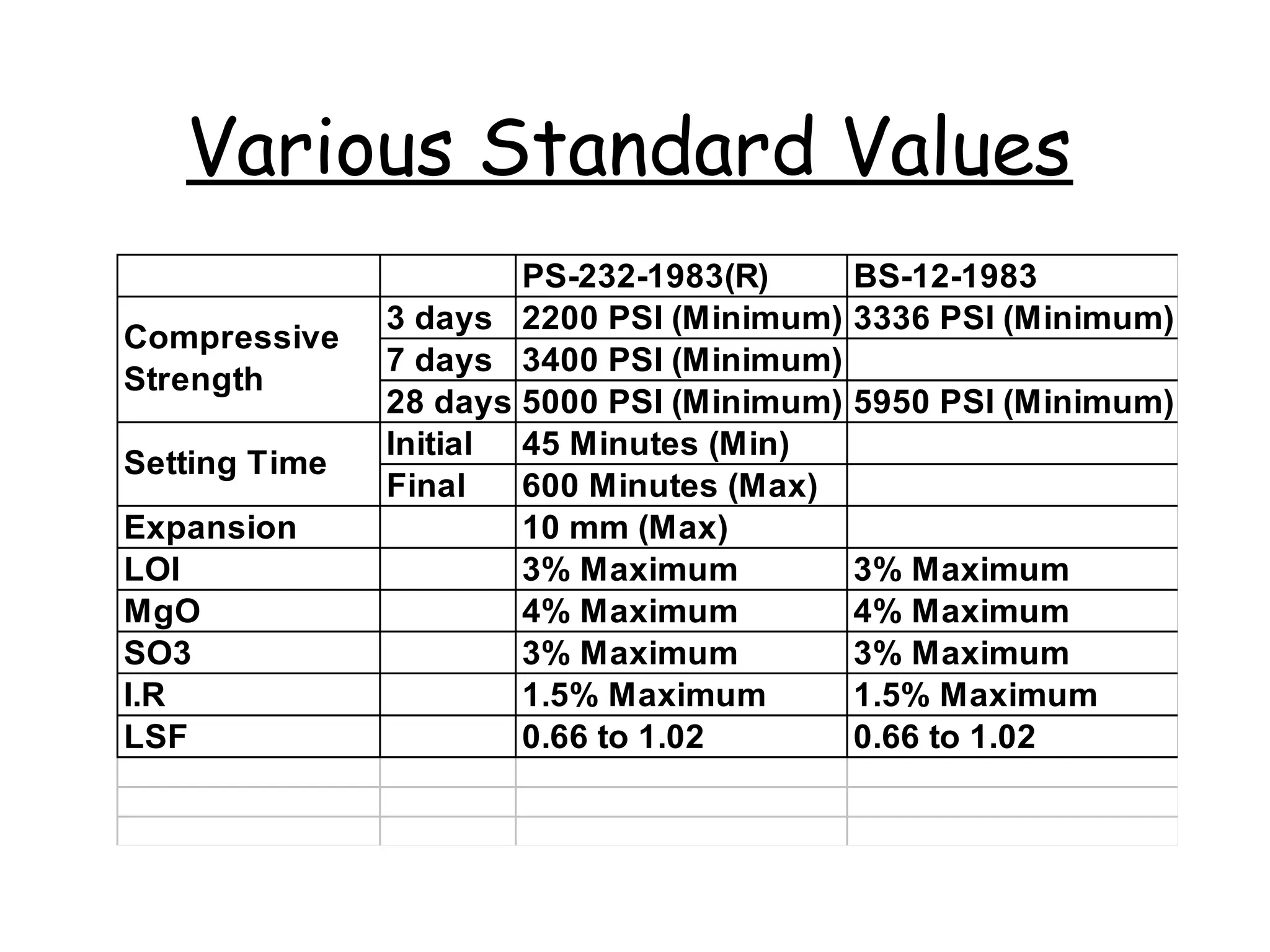
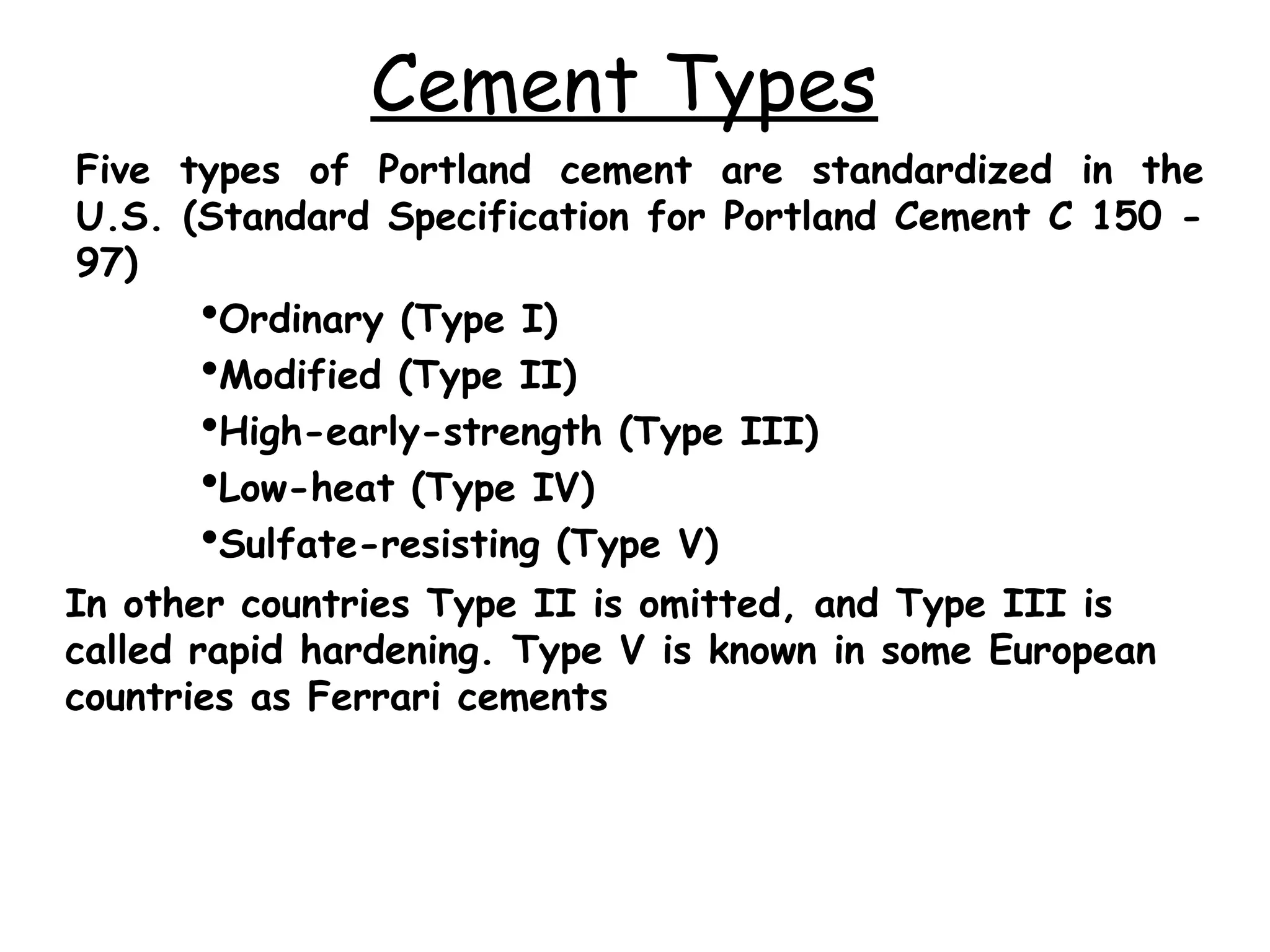
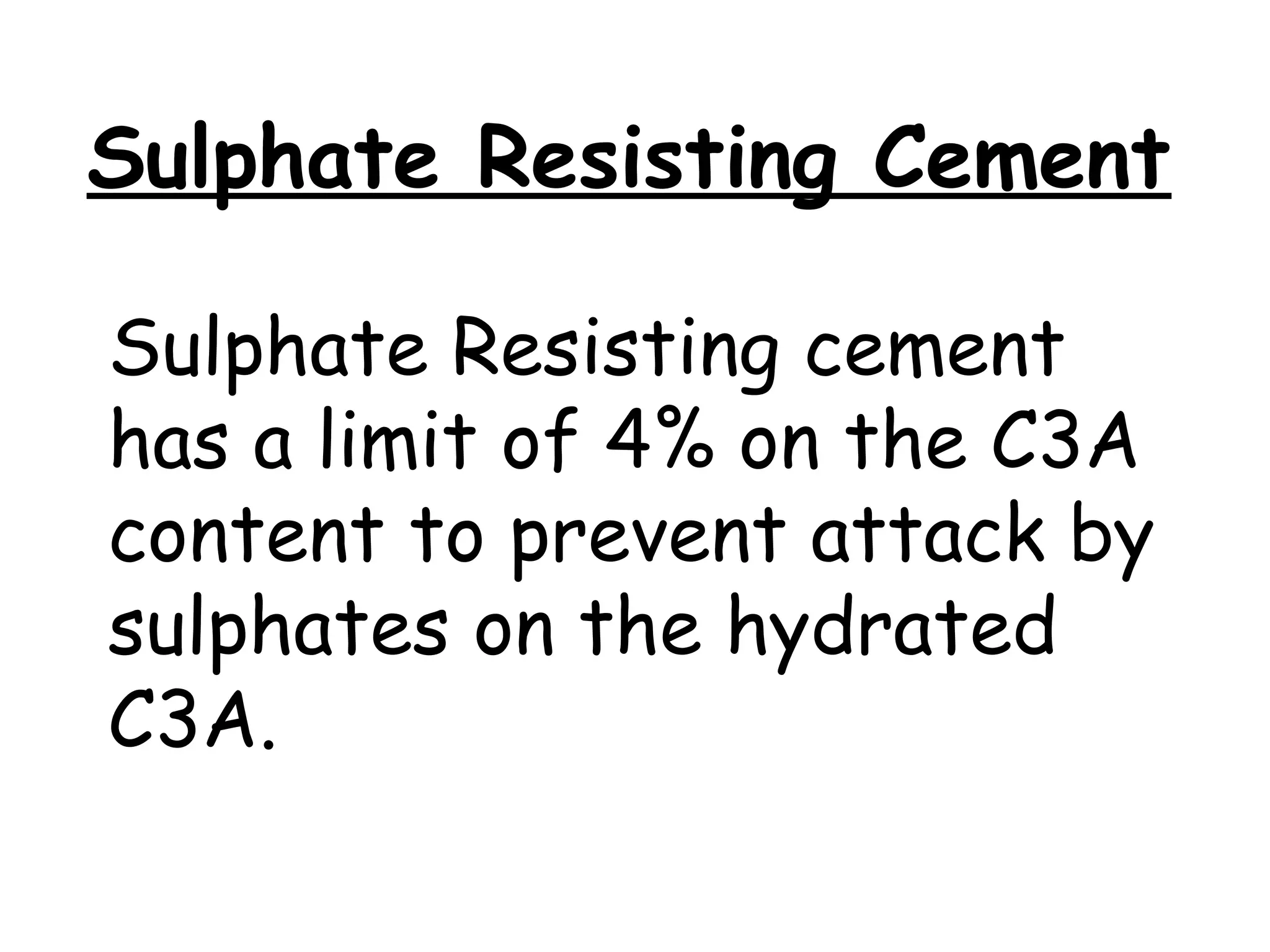
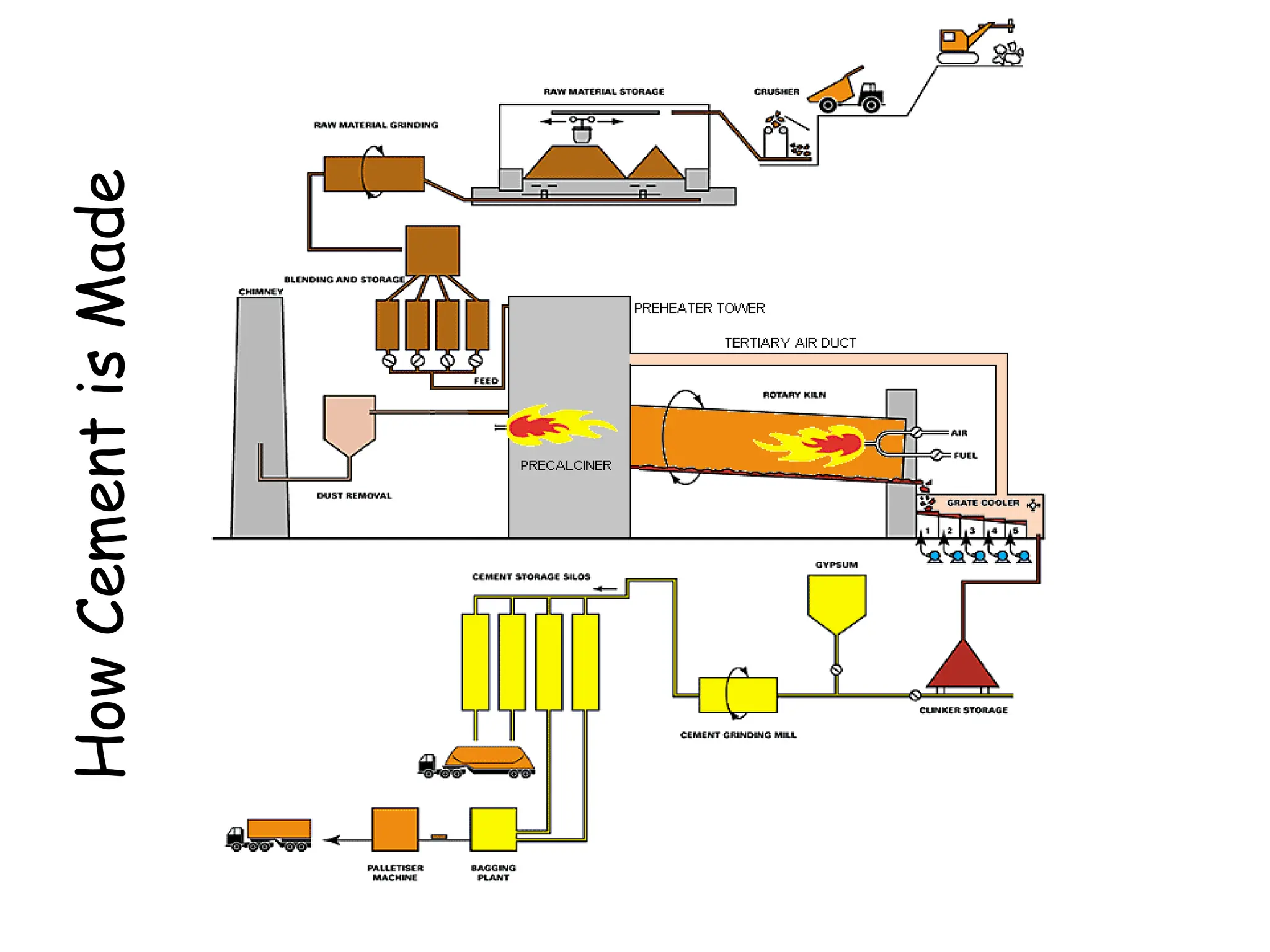
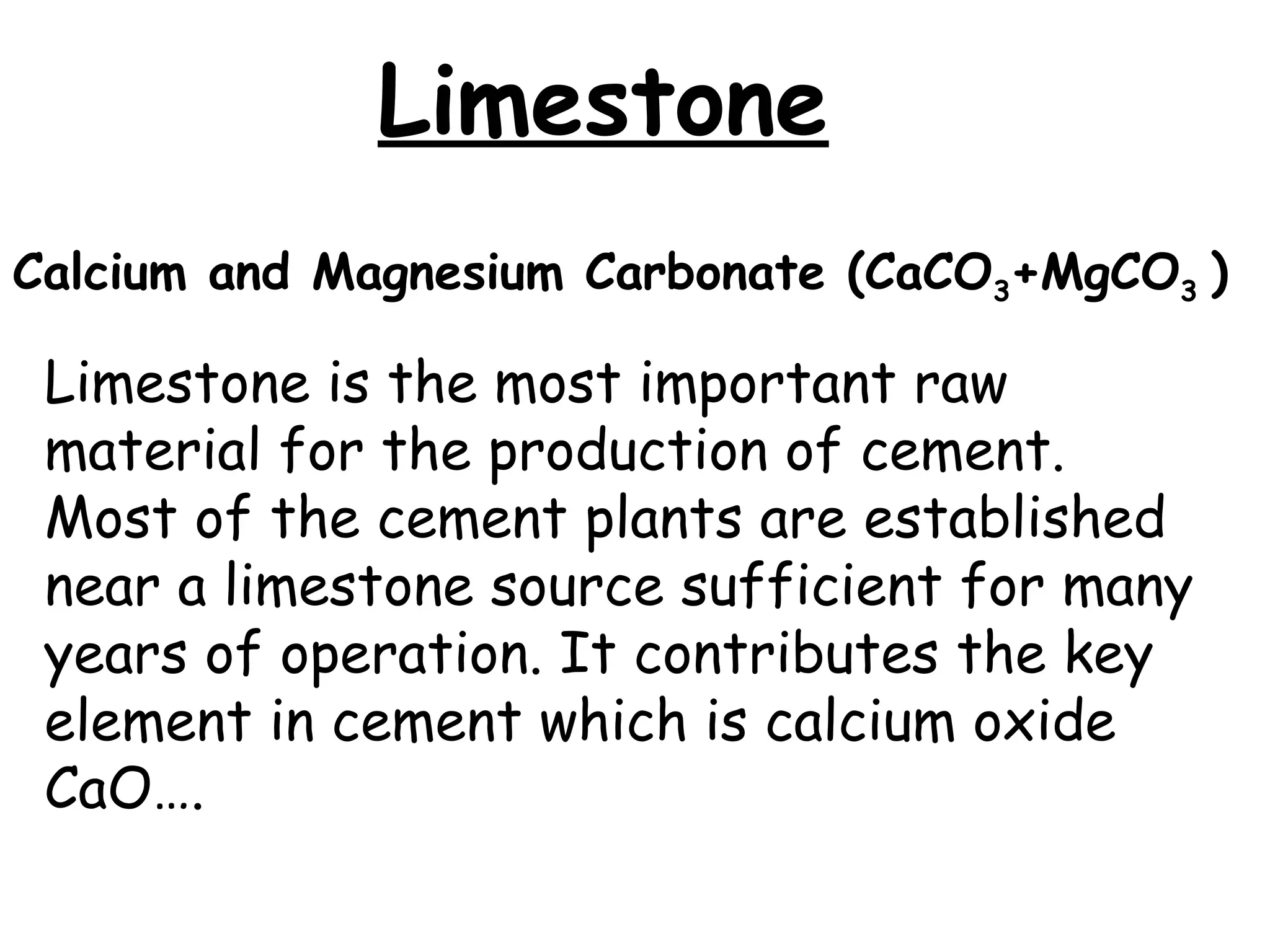
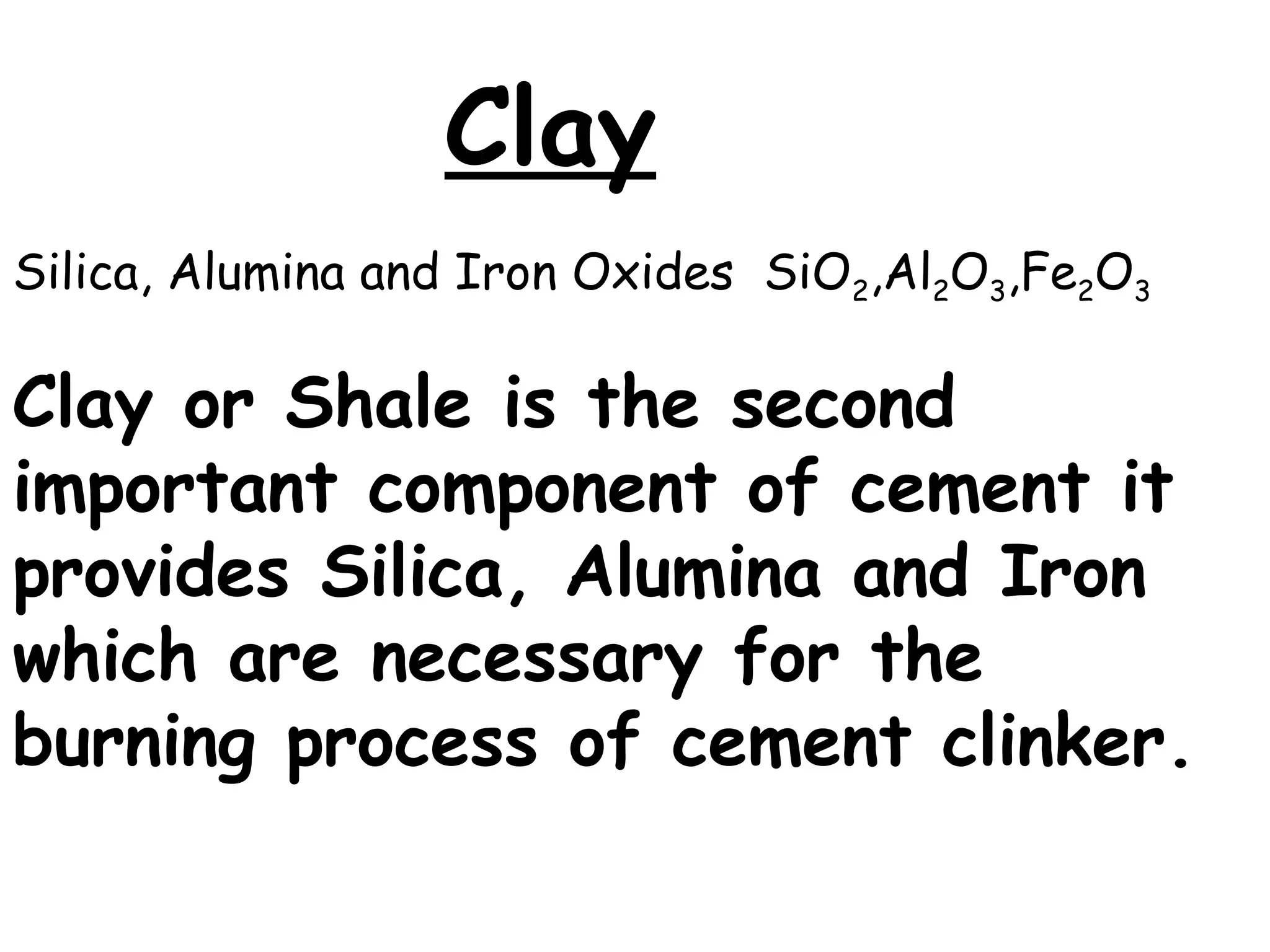
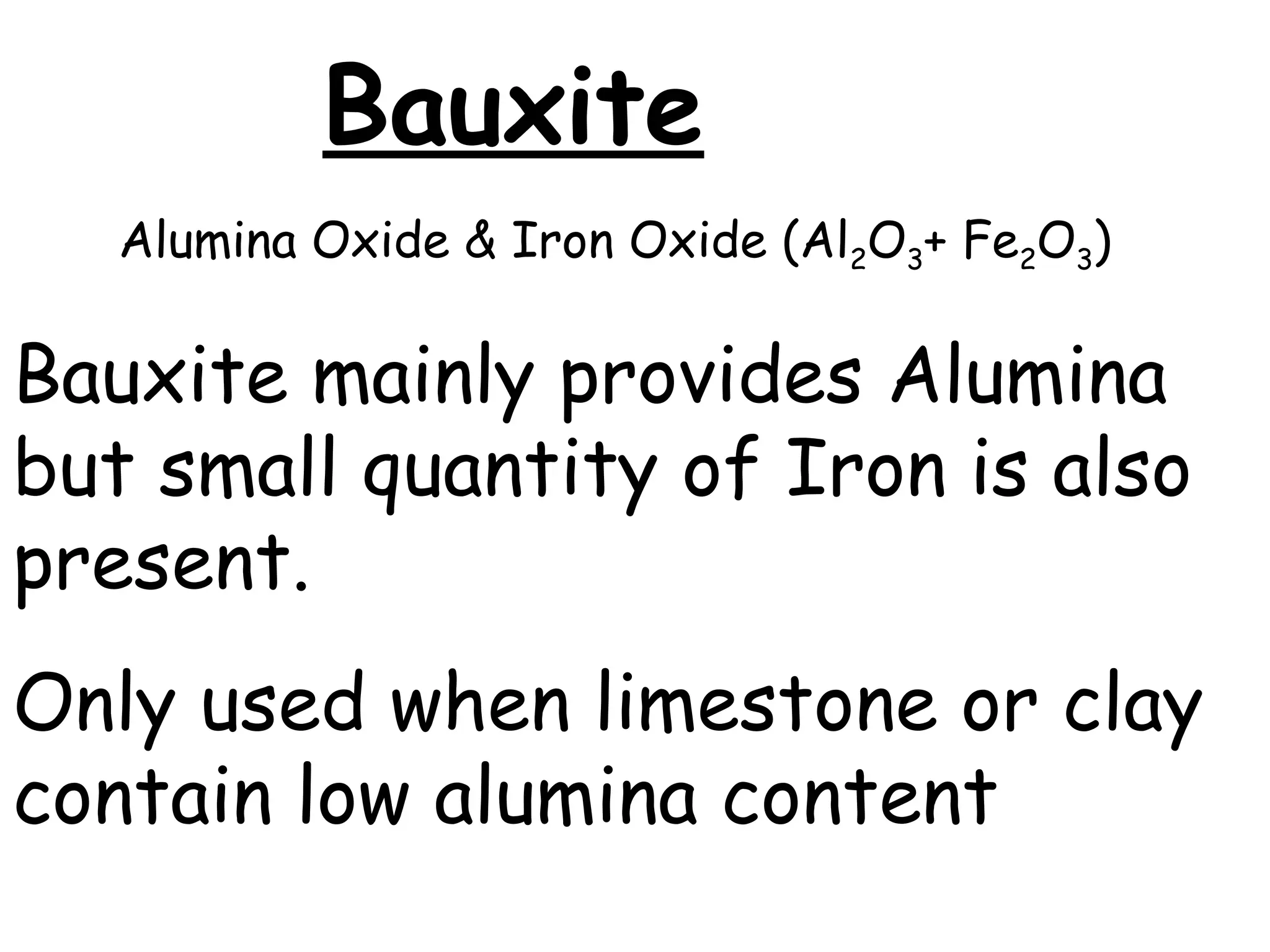
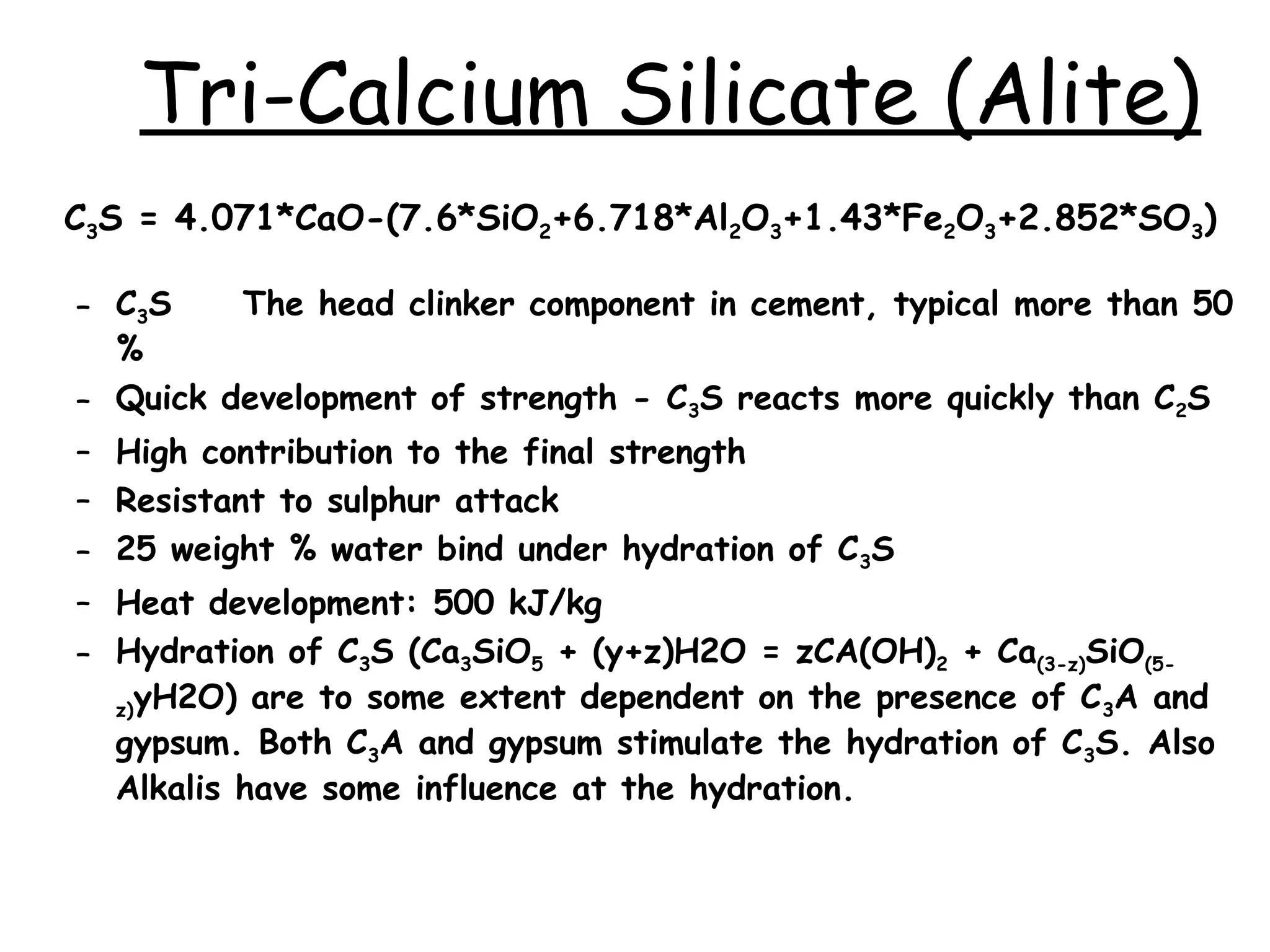
Cement is a hydraulic binder that hardens through hydration and retains strength even underwater, with Portland cement developed by Joseph Aspdin in 1824. There are various standardized types of Portland cement categorized based on their properties and applications, including ordinary, high-early-strength, and sulfate-resisting types. The manufacturing process involves grinding raw materials like limestone and clay, followed by burning them at high temperatures to form clinker, which is then ground into cement.