Embed presentation
Downloaded 69 times




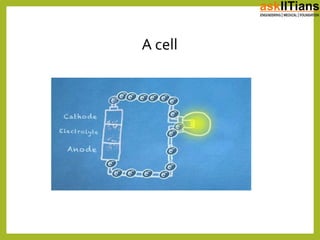

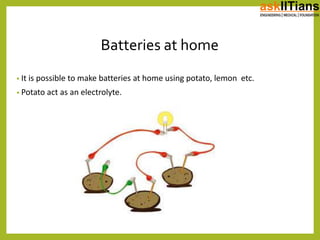
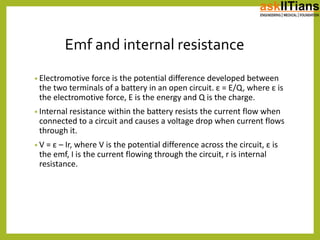
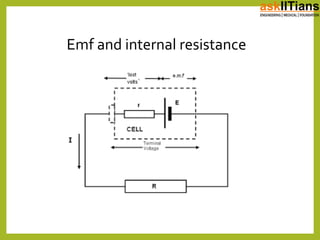

The document discusses the history of batteries and cells, including their key components and types. It explains that a cell consists of electrodes, electrolyte, and produces a chemical reaction. Primary cells cannot be recharged while secondary cells can be reused. The document also defines electromotive force (EMF) as the potential difference developed in an open circuit, and internal resistance as the resistance within a battery that causes a voltage drop under load. V=ε-Ir describes the relationship between these factors.









