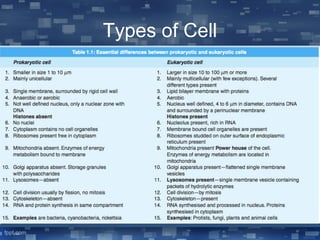
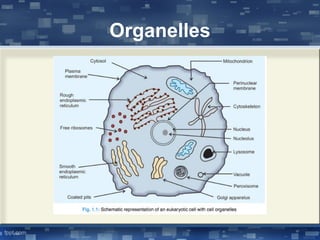
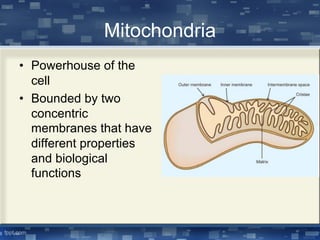
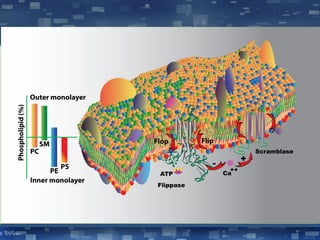
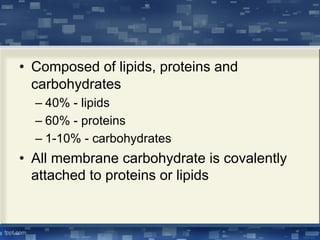
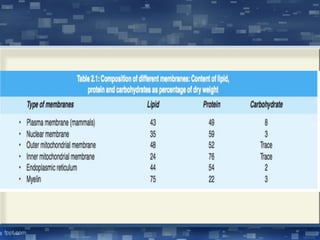
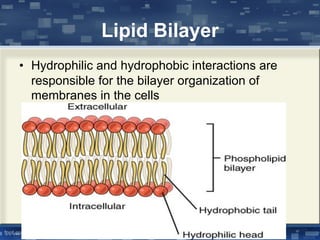
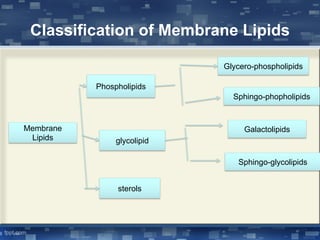
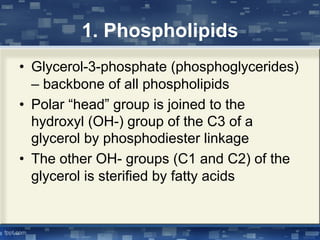
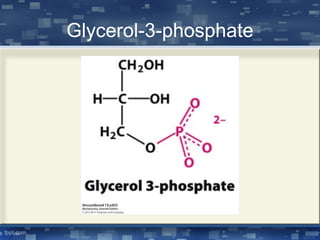
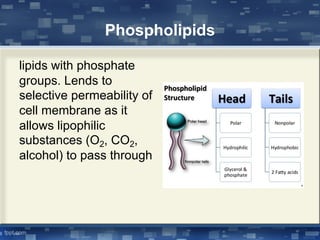
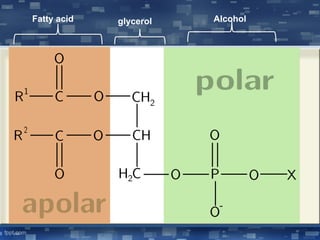
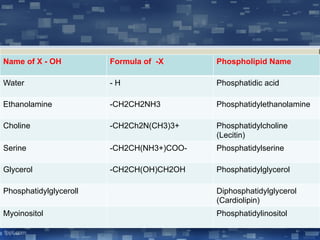
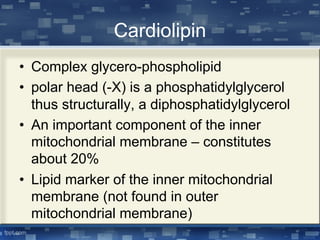
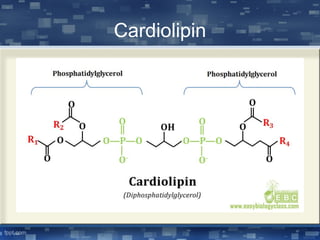
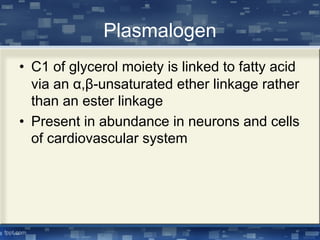
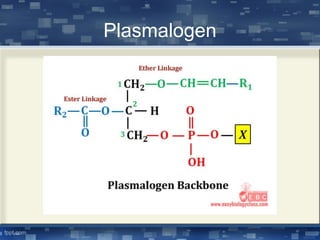
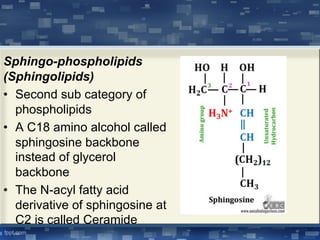
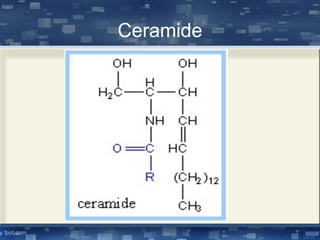
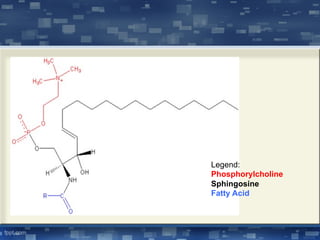
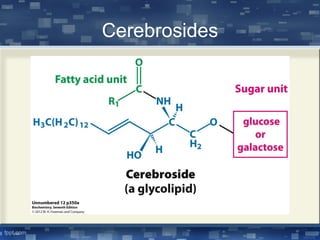
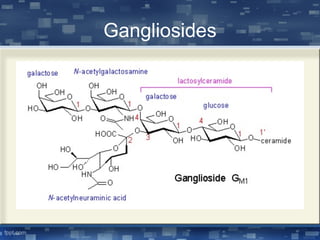
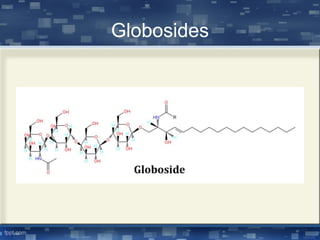
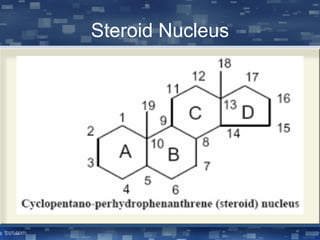
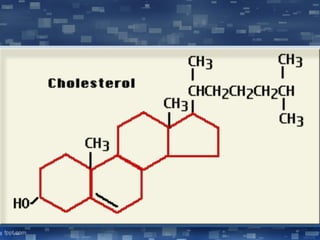
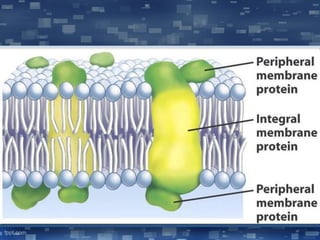
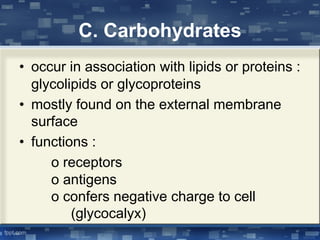
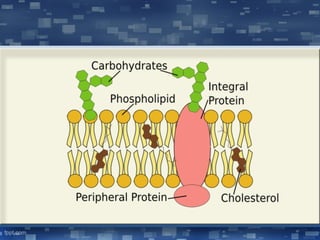
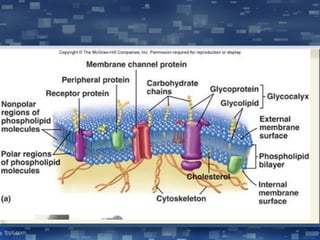
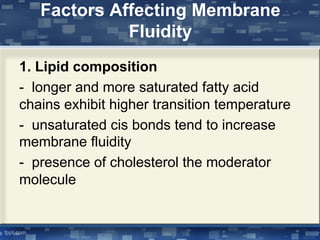
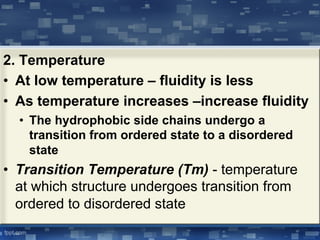
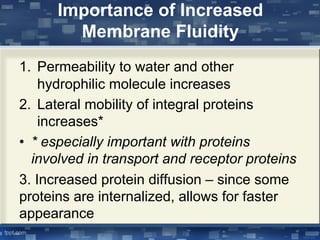
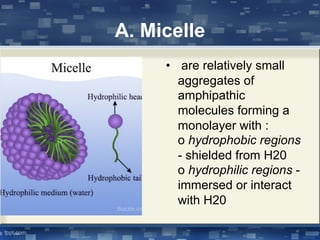
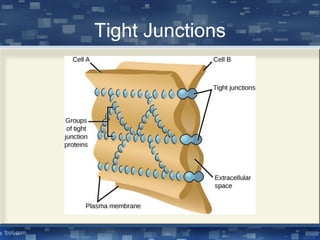
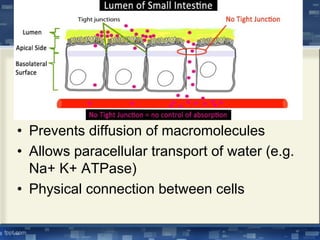
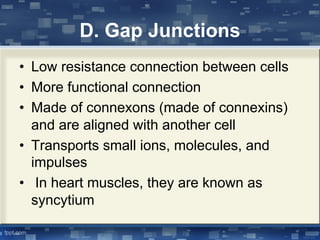
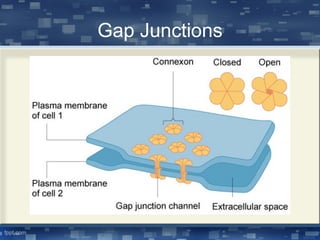
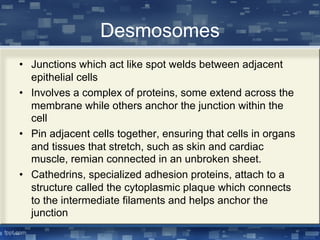
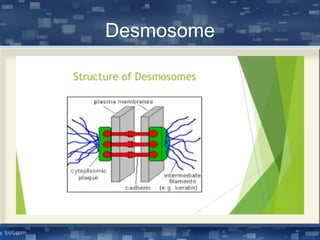
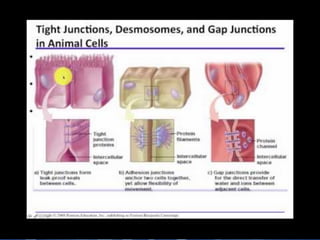
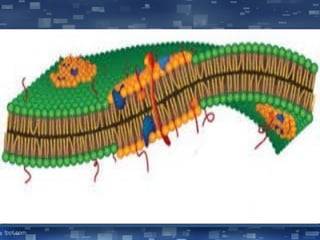
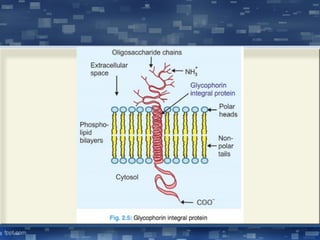
The document discusses cell membrane structure and function, emphasizing the role of various organelles such as the nucleus, mitochondria, endoplasmic reticulum, Golgi apparatus, lysosomes, and peroxisomes in cellular processes. It describes the composition of biological membranes, including phospholipids, glycolipids, sterols, and the function of membrane proteins in transport, enzymatic activity, and signaling. Additionally, it addresses membrane fluidity, the fluid-mosaic model, and the importance of cholesterol in maintaining membrane integrity.