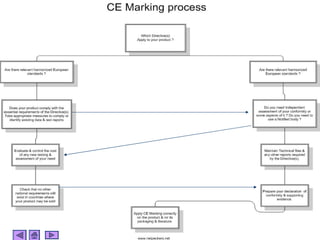
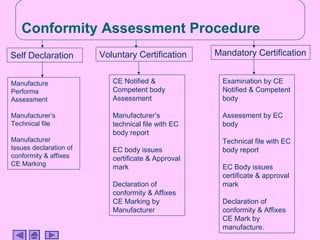
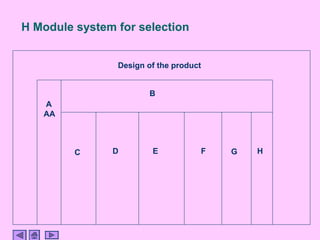
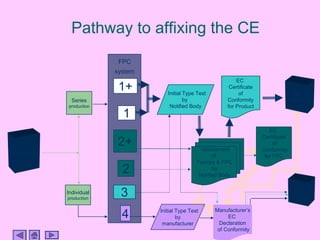
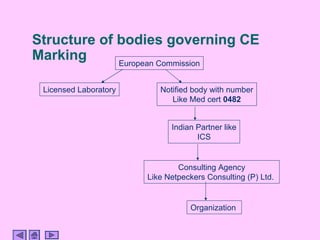
This presentation discusses CE marking and the process of implementing it. CE marking shows that a product has met EU health, safety, and environmental requirements. The presentation outlines the key directives, conformity assessment procedures, notified bodies, and steps to affix the CE mark. It also discusses assessing medical devices and deciding on rules based on factors like invasiveness and intended use. Attendees are encouraged to provide feedback to help improve future presentations.