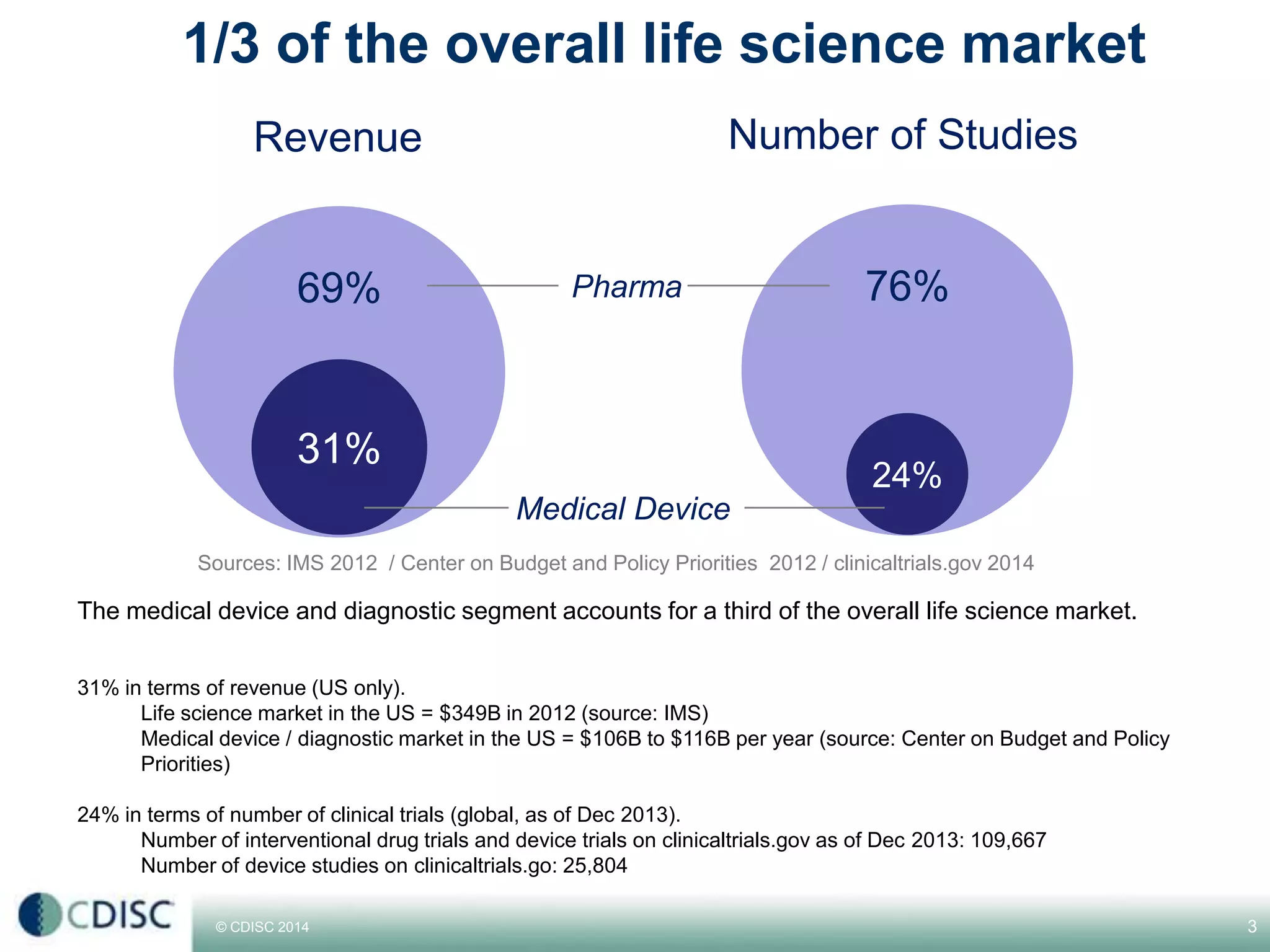
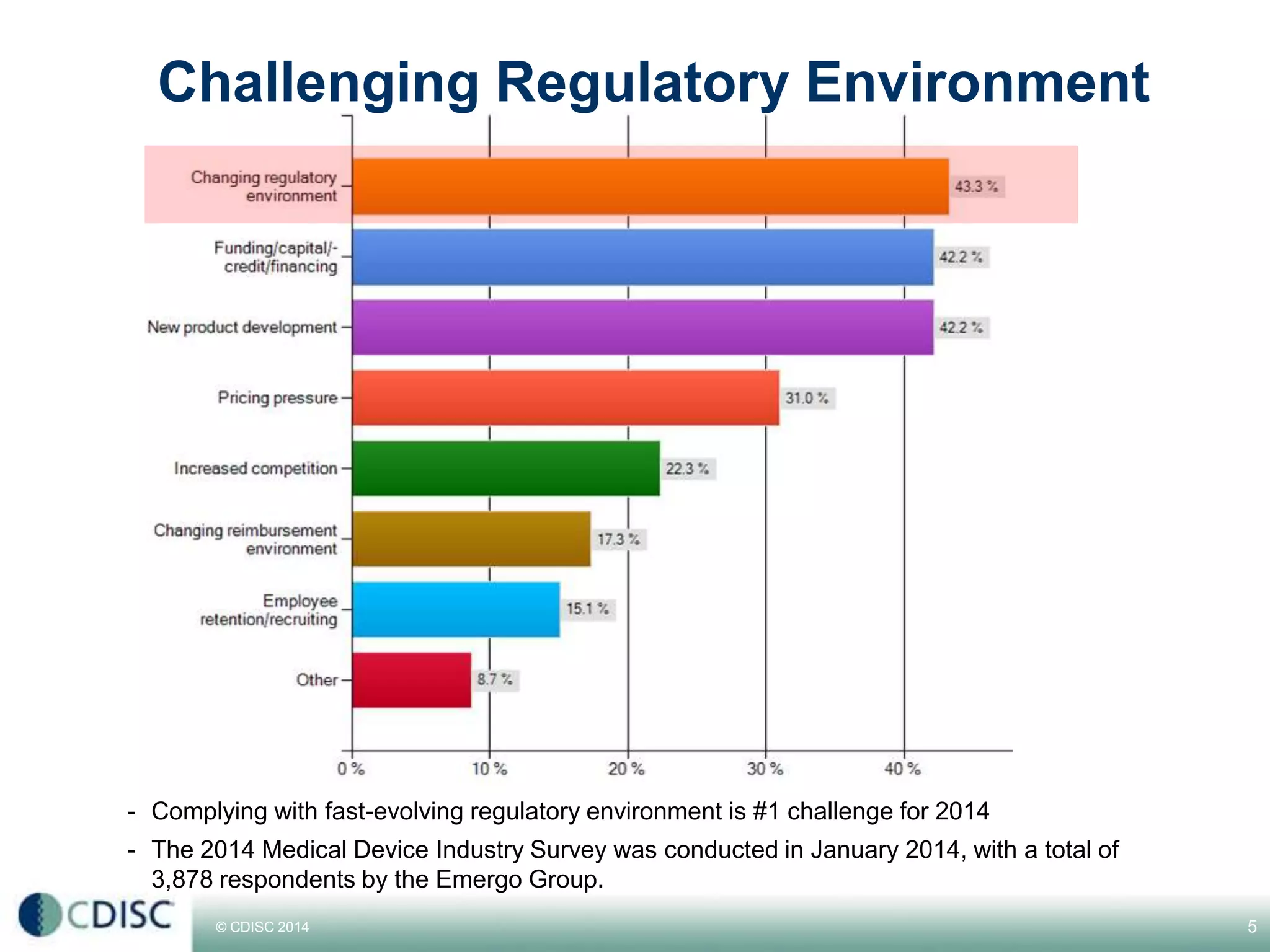
This document discusses strategies for medical device companies to adopt CDISC standards. It notes that while medical devices make up about 1/3 of the overall life sciences market in terms of revenue, they represent a larger number of studies than pharmaceuticals. It then presents 5 winning strategies for medical device companies to adopt CDISC standards: 1) Start with the team, 2) Make a plan from day one, 3) Prioritize, 4) Rely on experts, and 5) Leverage technology. The presentation concludes by envisioning a world where medical device data is increasingly standardized and shared using CDISC standards.