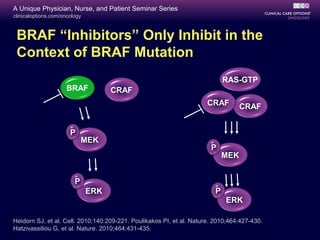
This document summarizes targeted therapy options for BRAF V600-mutant melanoma. Approximately 50% of melanomas harbor BRAF mutations. Clinical trials have shown that the BRAF inhibitors vemurafenib and dabrafenib, as well as the MEK inhibitor trametinib, provide significant improvements in response rates, progression-free survival, and overall survival compared to chemotherapy when used as initial therapies for BRAF V600-mutant metastatic melanoma. However, resistance to these targeted agents typically develops within 6-8 months. Mechanisms of resistance include bypass pathway activation or upregulation. Combination strategies of BRAF and MEK inhibitors are being studied to delay or prevent resistance.









![clinicaloptions.com/oncology
A Unique Physician, Nurse, and Patient Seminar Series
1. Chapman PB, et al. N Engl J Med. 2011;364:2507-2516. 2. McArthur G, et al. ECCO-ESMO 2011.
Abstract LBA28. 3. Hauschild A, et al. Lancet. 2012;380:358-365. 4. Robert C, et al. ASCO 2012.
Abstract LBA8509. 5. Flaherty KT, et al. N Engl J Med. 2012;367:107-114.
Phase III Trials in BRAF V600E–Mutant
Melanoma
Trial Name NCT Identifier N Treatment Arms
BRIM-3[1,2]
NCT01006980 675 Vemurafenib 960 mg PO BID (n = 337)
Dacarbazine 1000 mg/m2
IV q3w
(n = 338)
BRF113683[3]
NCT01227889 250 Dabrafenib 150 mg PO BID (n = 187)
Dacarbazine 1000 mg/m2
IV q3w (n = 63)
METRIC[4,5]
NCT01245062 322 Trametinib 2 mg PO QD (n = 214)
Dacarbazine1000 mg/m2
IV q3w or
paclitaxel 175 mg/m2
q3w (n = 108)](https://image.slidesharecdn.com/ccomelanoma-150422032129-conversion-gate02/85/Cco-melanoma-11-320.jpg)





![clinicaloptions.com/oncology
A Unique Physician, Nurse, and Patient Seminar Series
Most Common AEs With Approved
Targeted Agents in Advanced Melanoma
AE (≥ Grade 2), % Vemurafenib[1]
Dabrafenib[2]
Trametinib[3]
Arthralgia 21 5 NR
Rash 18 NR 27
Fatigue 13 6 9
Cutaneous SCC/
keratoacanthoma
12/8 6 (combined) NR
Hyperkeratosis 6 13 NR
Pyrexia NR 11 NR
Headache 5 5 NR
Photosensitivity (any grade) 12 3 NR
Hypertension NR NR 12
1. Chapman PB, et al. N Engl J Med. 2011;364:2507-2516. 2. Hauschild A, et al. Lancet. 2012;380:358-
365. 3. Flaherty KT, et al N Engl J Med. 2012;367:107-114.](https://image.slidesharecdn.com/ccomelanoma-150422032129-conversion-gate02/85/Cco-melanoma-17-320.jpg)