Case record...Spinal dural arteriovenous fistula with congestive myelopathy
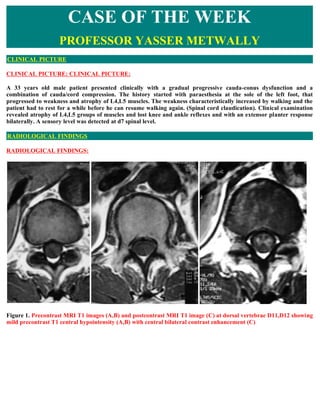
- 1. CASE OF THE WEEK PROFESSOR YASSER METWALLY CLINICAL PICTURE CLINICAL PICTURE: CLINICAL PICTURE: A 33 years old male patient presented clinically with a gradual progressive cauda-conus dysfunction and a combination of cauda/cord compression. The history started with paraesthesia at the sole of the left foot, that progressed to weakness and atrophy of L4,L5 muscles. The weakness characteristically increased by walking and the patient had to rest for a while before he can resume walking again. (Spinal cord claudication). Clinical examination revealed atrophy of L4,L5 groups of muscles and lost knee and ankle reflexes and with an extensor planter response bilaterally. A sensory level was detected at d7 spinal level. RADIOLOGICAL FINDINGS RADIOLOGICAL FINDINGS: Figure 1. Precontrast MRI T1 images (A,B) and postcontrast MRI T1 image (C) at dorsal vertebrae D11,D12 showing mild precontrast T1 central hypointensity (A,B) with central bilateral contrast enhancement (C)
- 2. Figure 2. MRI T2 images showing cord swelling at D11,D12 vertebrae with central hyperintensity taking the shape of the central grey matter Figure 3. Precontrast MRI T1 image (A) and postcontrast MRI T1 images (B,C). Before contrast (A) the spinal cord is enlarged and hypointese. After contrast injestion, dilated enhanced perimedullary veins were observed on the surface of the spinal cord (B,C)
- 3. Figure 4. MRI T2 imaging showing pencil-shape multisegmental central spinal cord hyperintensity extending from D6 to D12 vertebrae with cording swelling Figure 5. Lower dorsal type I dural spinal arteriovenous malformation Summary of the case 1. The onset in middle age suggests that SDAVF is an acquired condition, in contrast to intradural ventral fistulas or AVMs, which are assumed to be congenital abnormalities 2. An SDAVF is never located within the spinal parenchyma, in contrast to AVMs. 3. Patients with SDAVF very rarely present with spinal haemorrhage in contrast to patients with AVMs. 4. Associated vascular lesions are seen in AVMs, not in SDAVF. 5. Intradural AVMs occur much more often in the cervical region than SDAVF (Rosenblum et al., 1987).
- 4. 6. The increased pressure causes the venous system to ‘arterialize’, that is, the walls of intramedullary veins become thickened and also tortuous. The radicular feeding artery is often a dural branch and in a minority, the medullary artery. The shunt results in venous hypertension in the spinal cord, because the intramedullary veins and the radicular vein share a common venous outflow. The reduced arteriovenous pressure gradient (with the resultant of venous hypertension) results initially in congestive myelopathy (cord edema, swelling and cord petechial hemorrhages) that is responsible for the spinal cord swelling and central T2 hyperintensity, T1 central hypointensity. The central T2 hyperintensity took the shape of the central grey matter denoting the cord edema is primarily located in the central grey matter in congestive myelopathy due to spinal arteriovenous fistula probably because the central grey matter has the highest blood supply and the highest venous return. On long term basis (and with persistence of venous hypertension) a decrease in tissue perfusion might occur and might eventually result in venous infarction and spinal cord atrophy. (Hurst et al., 1995). DIAGNOSIS: DIAGNOSIS: TYPE I SPINAL DURAL ARTERIOVENOUS FISTULA WITH CONGESTIVE MYELOPATHY DEMONSTRATED AT THE LOWER DORSAL LEVEL DISCUSSION DISCUSSION: Spinal dural arteriovenous fistula (SDAVF) is a rare and enigmatic disease entity. The clinical features and structural changes have been recognized since 1926, and the pathophysiology and the essentials of treatment since 1974, but up to the present day it is unknown why these fistulas develop. The fistula between a radicular artery and the corresponding radicular vein within the dural root sleeve leads to congestion of the venous outflow of the spinal cord and eventually ischaemia. Patients, who are mostly middle-aged men, develop a progressive myelopathy, which at the early stages of the disease often mimics a polyradiculopathy or anterior horn cell disorder. By the time involvement of upper motoneurons or sacral segments makes the diagnosis of SDAVF inescapable, patients suffer from considerable neurological deficits. The diagnosis relies on MRI, which shows swelling of the spinal cord, with a centrally located hyperintense signal on T2-weighted images, and with hypointense ‘flow void’ phenomena dorsal to the cord, representing enlarged and tortuous veins. Catheter angiography is required to determine the exact location of the fistula as well as the angio-architecture, on which the mode of treatment depends. If the arterial feeder of the fistula is a tributary of the anterior spinal artery, embolization is not possible. After embolization recanalization may occur, but this is rarely seen after filling of the draining vein with glue. Alternatively, operation is a safe and permanent mode of treatment. No prognostic factors have been reliably established. Muscle strength and gait disturbances respond better to treatment than pain and symptoms related to damage of sacral segments. In any middle aged male patient with ascending motor or sensory deficits in the legs, SDAVF should be considered in order to prevent irreversible handicap. Up to the present day physicians continue to be perplexed by the extensive changes in structure and function caused by the development of an abnormal but often tiny connection between a radicular artery and a radicular vein, at some level along the spinal axis. First, it took several decades before the morphological changes in the spinal cord of patients who had died from the complications of progressive paraplegia were attributed not to an infectious or degenerative condition of spinal blood vessels but to venous congestion. Secondly, it is the caudal end of the spinal cord that is commonly first affected by congestive oedema and ultimately infarction, regardless of the level of the fistula, so that the initial clinical features often consist of sensory and motor symptoms ascending from the feet, suggesting a polyneuropathy or polyradiculopathy. Thirdly, treatment now largely consists of endovascular occlusion rather than surgical closure of the fistula, but the optimal techniques have not yet been found, since recurrences continue to occur. Finally, it is unclear which factors lead to the development of these fistulae and more specifically why they occur most often in middle-aged men. Foix and Alajouanine's description of the clinical and anatomical features It is not exactly known who gave the first description of a patient with what we now call spinal dural arteriovenous fistula (SDAVF). Retrospective interpretations depend on clinical features and sometimes on findings at autopsy, but
- 5. even then these represented only the consequences of the disease and not its cause. Early reports of patients with appropriate spinal vascular lesions may have been misinterpreted and perhaps remain to be detected. The first detailed clinical and pathological report of what is most likely to represent SDAVF is the detailed description by Foix and Alajouanine (Foix and Alajouanine, 1926), who have, in the course of time, become eponymously linked with the condition until its cause was found. In 1926, Foix and Alajouanine described first the clinical and then the post-mortem findings in two young men with an ascending myelopathy, who died 33 and 11 months after the onset of an ascending paraparesis. The first patient presented at age 29 with a 7-month history of progressive ‘claudication intermittente de la moelle’, which the patient first noted after climbing stairs. He had problems with micturition but no sensory symptoms. On examination he showed wasting of the thighs and buttocks, weakness of foot dorsiflexion and knee flexion, with an ankle clonus and brisk knee jerks but no Babinski signs. Within 1 year and 6 months after disease onset he was fully paraplegic. By that time sensory disturbances had also developed, first with loss of pain and temperature sensation in the buttocks and at the back of the legs, gradually ascending to the level of the groins for all modalities of skin sensation. He developed bedsores and constipation and eventually died, 2 years and 9 months after the onset of the disease. The second patient was a 27- or 37-year-old male plumber (there is some inconsistency about his age in the report) who experienced weakness of the legs after a day's work. Initially the weakness resolved but after a few months the legs became progressively weak. He had some difficulty voiding. On examination there was paraparesis with marked weakness of knee flexion; plantar reflexes and ankle jerks were absent, with normal knee jerks; sensation was unimpaired. The disease progressed and 8 months after disease onset there was a complete and flaccid paraplegia, with marked wasting (especially on the right), areflexia (including abdominal and cremasteric reflexes), loss of pain and temperature sensation below the umbilicus, with touch first impaired only in the right foot and leg, and later also in all regions below the umbilicus. By then he had also urinary incontinence and constipation. He developed bedsores and died 11 months after the onset of the disease. Post-mortem examination was remarkably similar in the two patients. The lower spinal cord showed extensive necrosis, predominantly in the grey matter but also in the white matter (Fig. 1). The abnormalities were most severe at the sacral level and slightly less severe at the lumbar level, and they gradually disappeared at the mid-thoracic level, with the exception of secondary degeneration of long tracts in the white matter. The anterior and, to a lesser degree, posterior spinal roots were degenerated. Even more conspicuous were the changes of blood vessels. The intrinsic as well as the extramedullary vessels were thickened, through hypertrophy of the intimal and muscular layers (‘endo-méso-vascularite’), as well as widened and tortuous; the lumen had increased rather than decreased (Fig. 2). The abnormal blood vessels were found from the cauda equina up to the upper thoracic cord, where the parenchymal changes were only minimal. Therefore, the authors supposed that the vascular alterations had preceded the necrotic changes.
- 6. Figure 1. Lumbosacral spinal cord of Patient 1 (Weigert stain); ventral aspect at top of picture. Complete necrosis of the grey matter; no nerve cells can be identified. Less severe changes in the white matter, predominantly in the lateral and dorsal columns; relative sparing of the ventral and anterolateral columns. Dilatation and hypertrophy of intramedullary and extramedullary vessels may be noted. (Reproduction of Fig. 12 of the publication by Foix and Alajouanine in 1926.) Figure 2. Dorsal aspect of spinal cord of Patient 2 (stain not recorded, probably haematoxylin– eosin). Typical ‘endo- mesovasculitis’ with hypertrophic, ‘onion-bulb’ aspect. The adjacent parenchyma shows severe necrotic changes. (Reproduction of Fig. 28 of the publication by Foix and Alajouanine in 1926.) They were in the dark about the cause of the disease but hypothesized an infectious origin, primarily affecting the spinal blood vessels or involving both vascular and neural structures; also, they proposed future experiments in which
- 7. a spinal cord emulsion might be injected into an animal to see whether the disease was transmissible. Sub-acute necrotizing myelopathy probably exists in other forms, without concomitant vascular abnormalities. There have been reports of patients who suffered from a progressive myelopathy, clinically resembling the cases described by Foix and Alajouanine, but who did not have enlarged vessels at autopsy (Hoffman, 1955; Katz and Ropper, 2000). Subsequent history The observations from Foix and Alajouanine were confirmed 5 years later by Lhermitte et al. (1931). They described a 50-year-old man who presented with slowly progressive right-sided leg weakness. When the disease progressed further he was operated for a suspected spinal cord tumour. At operation they found multiple, dilated vessels on the dorsal surface of the spinal cord. As the expected spinal cord tumour was not found, the operation was terminated. After the operation the patient became paraplegic and ultimately died of urinary tract infection. Post-mortem examination again showed multiple, enlarged vessels and necrosis of the grey and white matter of the spinal cord. Probably the first successful operation on an SDAVF was performed by Elsberg, as early as in 1916 (Elsberg, 1916). He ligated and excised an enlarged and thickened vein at the level of T8 in a patient with a sensory level at T9. The patient made a full recovery. Elsberg also reported that in 120 laminectomies for other reasons he had found enlarged veins on the dorsal surface of the spinal cord in six patients (Elsberg, 1916). In a 1943 monograph on vascular abnormalities and tumours of the spinal cord the author lamented that ‘the subject is clouded by a loose and confusing nomenclature’ (Wyburn-Mason, 1943). Indeed many names have been proposed for the disorder, before and since. Apart from Foix and Alajouanine's ‘myélite nécrotique subaiguë’, SDAVF has been called angiodysgenetic necrotizing myelopathy (Scholz and Wechsler 1959; Jellinger et al., 1968), angioma racemosum venosum (Krause, 1911), dorsal extramedullary angioma, long dorsal AVM (Malis, 1982), type 1 arteriovenous fistula and venous angioma (Pia and Vogelsang, 1965). Wyburn-Mason was not far from the truth in his presumption that the cases described by Foix and Alajouanine represented a complication of an angioma. After the introduction of catheter angiography in the 1950s, knowledge about spinal AVMs and fistulas increased at a fast pace. The first somewhat larger series (15 patients) of angiographically demonstrated spinal vascular lesions (through vertebral arteriography, via the subclavian route, or aortography) was published in 1966 (Houdart et al., 1966). The authors distinguished spinal vascular lesions on the basis of the angioarchitecture of the malformation, arterial supply and draining veins, location of draining veins and width of the lesion. They did not provide sufficient clinical data to allow an estimation of the number of spinal fistulas in their series. In 1972, Djindjian was the first to recognize that some arteriovenous malformations (AVMs) consist of an arteriovenous shunt without an intervening capillary plexus (Djindjian, 1972). The first embolization of an AVM—a congenital condition, not a fistula—was performed by Doppman et al. (1968) in a 16-year-old boy, by means of six stainless steel metal pellets, 1.5 mm in diameter. The pathophysiology of ‘necrotizing myelopathy’ continued to be poorly understood until 1974, when Aminoff and Logue at the National Hospital for Nervous Diseases (as it was then called) hypothesized that the arteriovenous shunt leads to increased intramedullary venous pressure, with a reduced arteriovenous pressure gradient (Aminoff et al., 1974). They described a 49-year-old patient who had died after a 12-year history of progressive, bilateral leg weakness. Post-mortem examination showed ischaemic changes of the spinal cord without features of thrombotic occlusion of vessels. In addition, there was extensive concentric hyalinization of the intramedullary capillaries. Given the absence of thrombotic occlusion they attributed these changes to increased intramedullary pressure. Although a landmark article, it was not the first to assume venous hypertension as the pathophysiological factor behind the thickened venous walls. Antoni wrote from Sweden in 1962: ‘In any event they (the thick walled and enlarged vessels) indicate a pronounced, longstanding increase of intravascular pressure, of a type which occurs primarily between the arterial and venous systems’ (Antoni, 1962). In 1976, Aminoff published a monograph on spinal angiomas, with more details on the natural history, presumed pathogenesis of venous congestion and the surgical treatment, which then consisted in excision of the draining veins (Aminoff, 1976).
- 8. Finally, in a beautifully illustrated article in 1977 Kendall and Logue showed that the site of the fistula was not located in the spinal cord but on or in the dural root sleeve (Kendall and Logue, 1977). Classification Several classification systems have been used in the past for spinal vascular lesions (Wyburn-Mason, 1943; Pia and Vogelsang, 1965; Rosenblum et al., 1987; Borden et al., 1995; Spetzler et al., 2002). The author prefers the classification system described by Spetzler et al. (Table 1), because it encompasses the cumulative knowledge thus far about the angioarchitecture of the vascular lesions. Like every classification system it has its drawbacks: the designation of cavernous malformations as neoplastic is disputable, while the classification system contains a new entity called conus medullaris AVM that had not been described before (Barrow, 2002). Table 1. Classification of spinal vascular lesions (Spetzler et al., 2002) Neoplastic vascular lesions Haemangioblastoma Cavernous malformations Aneurysms Arteriovenous lesions Arteriovenous malformations Arteriovenous fistulas Extradural Intradural Ventral A: Small B: Medium C: Large Dorsal A: Single arterial feeder B: Multiple arterial feeders According to the Spetzler classification, spinal vascular lesions can be subdivided into neoplasms, aneurysms and arteriovenous lesions (Table 1). Arteriovenous lesions are further classified as arteriovenous fistulas and AVMs. Arteriovenous fistulas can either be extradural or intradural. Extradural arteriovenous fistulas have also been called epidural fistulas and consist of a shunt between an extradural artery and vein. This is a high-flow fistula, which in turn is responsible for enlargement of the epidural venous plexus, leading to external compression of the spinal cord. Myelopathy may occasionally develop because of vascular steal (Arnaud et al., 1994a; Goyal et al., 1999). Intradural arteriovenous fistulas are the typical lesions causing progressive myelopathy. They may be located ventrally or dorsally. Ventral intradural arteriovenous fistulas consist of a shunt between the anterior spinal artery and an enlarged venous draining system (Djindjian et al., 1977). They are subdivided into type A, B or C, depending on the size. Ventral intradural lesions were formerly known as type IV lesions (Heros et al., 1986; Barrow et al., 1994); with dorsal intradural arteriovenous fistulas (SDAVF) the abnormal connection is formed between an artery and vein at the level of a dural root sleeve, with low flow, which is in contrast to the high-flow ventral type of fistulas. The dorsal fistulas can be subdivided into fistulas with a single feeding artery (dorsal type A) and those with multiple feeding arteries (dorsal type B) (Spetzler et al., 2002). In this article, we will only deal with dorsal fistulas, further called
- 9. SDAVF. Another classification system, less static because it is not based on descriptive morphological features, is the system that divides spinal cord vascular lesions into three main groups (Berenstein et al., 2004). The first group consists of single shunts that are caused by a genetic disorder, for example arteriovenous lesions associated with hereditary haemorrhagic telangiectasia (Rendu–Osler–Weber disease). The second group includes multiple spinal cord vascular lesions that are not genetically determined but share metameric links (involvement with cord, bone, paraspinal, subcutaneous and skin tissues). The third group consists of single lesions, which are either AVMs or arteriovenous fistulas. Epidemiology SDAVFs are rare, but they still make up the most common vascular anomaly of the spine, with a proportion of 60– 80% (Kendall and Logue, 1977; Merland et al., 1980; Oldfield and Doppman, 1988). Absolute figures are not available, but an estimation based on retrospective analysis of German patients with a progressive myelopathy arrived at 5–10/million/year in the general population (Thron, 2001). The disease is probably underdiagnosed (Grandin et al., 1997). Jellema et al., 2006 addressed the question whether undiagnosed SDAVF occurred in patients who were admitted to a specialized rehabilitation institute for patients with spinal cord lesions. Of 614 patients who suffered from a spinal cord lesion not due to trauma Jellema et al., 2006 found at least three patients with either a SDAVF or a cerebral fistula with spinal drainage (by extrapolation there might have been three more if the films and records had not been destroyed in the mean time) (Jellema et al., 2006). None of them had been previously recognized as suffering from SDAVF. One patient was diagnosed only after post-mortem examination (Fig. 3). Figure 3. (A) Spinal cord of a patient with an SDAVF demonstrated at autopsy. The lower panel shows marked necrosis and enlarged vessels at the thoracolumbar spinal cord. The panel above shows a normal cord. (B) Gross macroscopic view of the same patient. Patients affected by SDAVF are mostly middle-aged men. Table 2 lists all reported series with more than 5 patients; there were 968 men against 210 women (ratio almost 5 : 1). The mean age at the time of diagnosis is 55–60 years (see Table 2); patients under the age of 30 are rarely reported (in total 14 patients under age 30 were found in the 1178 patients, or 1%) which is in contrast to the mean age of 30 in patients with an intramedullary AVM (Oldfield et al.,
- 10. 1983; Symon et al., 1984; Rosenblum et al., 1987; Bedersen and Spetzler, 1996; Niimi et al., 1997; Kataoka et al., 1999; Sleiman et al., 1999; Lev et al., 2001; Van Dijk et al., 2002). The youngest patients reported were 22 years at the time of diagnosis (Rosenblum et al., 1987; Bedersen and Spetzler, 1996). The archetypal patients described by Foix and Alajouanine were also rather young in comparison, being 29 and (probably) 37 years at the onset of the disease (Foix and Alajouanine, 1926). In our series of 80 patients the youngest patient developed symptoms of SDAVF under the age of 30, but he was not diagnosed until the age of 34 (Jellema et al., 2003). No patient under the age of 20 has ever been reported. Table 2. Gender, location and diagnostic delay in reported series of patients with SDAVF reporting more than five patients Author (year) Men Women Mean Range Cervical Sacral Delay (in Multiple age months) SDAVF Afshar et al. (1995) 17 2 56 29–70 0 1 22 0 Arnaud et al. (1994) 7 1 66.5 51–80 0 0 14 0 Atkinson et al. (2001) 75 19 63 31–81 1 7 23 0 Bedersen and Spetzler (1996) 3 9 51 22–75 0 0 34 0 Behrens and Thron (1999) 18 3 57 33–75 0 1 15.5 0 Berenstein and Lasjaunias 26 5 56 35–87 n.d. 0 (1992) Bowen et al. (1995) 6 2 60 45–71 0 0 n.d. 0 Bradac et al. (1993) 13 0 41 30–71 0 2 17 0 Cenzato et al. (2004) 29 8 57 24–70 1 0 22 Coats and King (1991) 6 1 69 67–72 0 0 44 0 Brunereau et al. (1996) 5 2 61 44–79 n.d. n.d. 26 0 Criscuolo et al. (1989) 5 0 61 56–68 0 0 n.d. 0 Djindjian (1976) 38 6 52 34–73 n.d. 0 Eskandar et al. (2002) 22 4 65 39–79 5 3 21 0 Gilbertson et al. (1995) 51 15 62 37–81 5 6 27 0 Guillevin et al. (2005) 22 4 62 38–87 n.d. n.d. 25 0 Huffmann et al. (1995) 19 2 58 38–78 1 2 14 0 Jellema et al. (2003) 66 14 60 34–79 2 3 15 3 Kataoka et al. (2001) 3 4 47 25–58 0 0 39 0 Koch et al. (2003) 42 12 60 35–83 4 4 20 0 Koenig et al. (1989) 18 2 54 34–71 0 1 34.5 0 Lee et al. (1998) 7 2 61 40–73 0 0 38 0 Lev et al. (2001) 8 1 60 46–75 0 0 16 0 Li et al. (2005) 61 11 56 28–72 n.d. n.d. 16 0 Linden and Berlit (1996) 7 4 60 32–81 0 1 28 0
- 11. Logue (1979) 21 3 57.5 33–73 n.d. n.d. 22 0 Lundqvist et al. (1990) 8 3 61 44–70 0 0 25.5 0 Mascalchi et al. (1995) 6 0 64 43–75 0 0 n.d. 0 Merland et al. (1980) 10 3 56 39–72 0 1 n.d. 0 Morgan et al. (1989) 12 5 60 51–72 0 0 24 0 Mourier et al. (1989) 27 3 60 34–77 0 1 12 0 N'Diaye et al. (1984) 22 3 62 32–79 0 0 n.d. 0 Nichols et al. (1992) 9 5 63 51–74 0 0 21 0 Niimi et al. (1997) 42 7 60 26–82 0 9 n.d. 0 Oldfield et al. (1983) 6 0 56 29–74 0 0 33 0 Rosenblum et al. (1987) 23 4 49 22–72 0 1 32 0 Schick and Hassler (2003) 16 2 60 32–84 n.d. n.d. n.d. 0 Sleiman et al. (1999) 8 2 60 24–75 0 0 23 0 Song et al. (2001) 27 5 65 46–83 n.d. n.d. 29 0 Symon et al. (1984) 53 2 57 29–75 0 2 n.d. 0 Tacconi et al. (1997) 21 4 63.1 42–78 0 0 33 0 Ushikoshi et al. (1999) 9 4 57 38–73 2 0 23 0 Van Dijk et al. (2002) 39 10 63 28–78 1 1 27.6 1 Westphal and Koch (1999) 35 12 60 35–? 1 1 24 2 Most SDAVFs are located in the thoracolumbar region. In the tabulated series with more than 5 patients Jellema et al., 2006 found 23 patients with cervical SDAVF (2% of total) and 47 patients with SDAVF in the sacral region (4% of total). Together, cervical and sacral SDAVF constitute just under 6% (70 of 1178) of patients with SDAVF. Multiple SDAVFs in a single patient are uncommon (Table 2). In the 1178 patients listed in Table 2 they were encountered six times (0.5%). Yet this is probably an underestimation, because spinal angiography is mostly terminated after a single fistula has been found. Two series reported one patient (2%) and two patients (4%) with a double SDAVF among almost 50 patients (Westphal and Koch 1999; Van Dijk et al., 2002). Apart from the series with more than five patients listed in Table 2, four case reports described five patients with a double SDAVF (Barnwell et al., 1991; Pierot et al., 1993; Chaloupka et al., 1995; Krings et al., 2004). Causal factors and pathophysiology The onset in middle age suggests that SDAVF is an acquired condition, in contrast to intradural ventral fistulas or AVMs, which are assumed to be congenital abnormalities (Rosenblum et al., 1987). There are several other differences between SDAVF and AVMs. An SDAVF is never located within the spinal parenchyma, in contrast to AVMs. Patients with SDAVF very rarely present with spinal haemorrhage in contrast to patients with AVMs. Associated vascular lesions are seen in AVMs, not in SDAVF. Intradural AVMs occur much more often in the cervical region than SDAVF (Rosenblum et al., 1987). In cerebral dural fistulas a strong association exists with cerebral vein thrombosis (Tsai et al., 2004); also, an association with factor V Leiden and protein C has been demonstrated (Kraus et al., 1998; Kraus et al., 2000). This association with thrombophilia has not been found in patients with SDAVF (Jellema et al., 2004a).
- 12. Trauma does not seem to play a major role. First, in a retrospective study trauma to the spine was reported in only 4% of patients (Jellema et al., 2003). Secondly, although the cervical spine is the most mobile part of the spinal column, cervical fistulas are rare. Another proposed factor is partial thrombosis of an AVM (Brion et al., 1952), but no evidence for this has been put forward since. Since the introduction of selective angiography, much has been learned about the pathophysiology. Aminoff and others proposed in 1974 that venous hypertension and not vascular steal, cord compression or haemorrhage was the main pathophysiological factor (Aminoff et al., 1974). The shunt is most often formed within the dorsal surface of the dural root sleeve in the intervertebral foramen, where the radicular vein pierces the dura, together with one or more dural branches of the radicular artery. However, the shunt is sometimes situated along the dura between two adjacent nerve roots (Berenstein et al., 2004). The increased pressure causes the venous system to ‘arterialize’, that is, the walls of intramedullary veins become thickened and also tortuous. The radicular feeding artery is often a dural branch and in a minority, the medullary artery. The shunt results in venous hypertension in the spinal cord, because the intramedullary veins and the radicular vein share a common venous outflow. The reduced arteriovenous pressure gradient results in a decrease in tissue perfusion and venous infarction (Hurst et al., 1995). This has been confirmed by direct intra-operative measurement of vascular pressure of the fistula, which was as high as 74% of the systemic arterial pressure (Hassler et al., 1989; Hassler and Thron, 1994). An increase in arterial pressure during the operation directly leads to an increase in venous pressure (Hassler et al., 1989), which may explain why some patients report that symptoms become worse after physical activity (Aminoff and Logue, 1974a; Khurana et al., 2002). Apart from the increased pressure caused by the shunt, the venous outflow may be less efficient to start with than is the case in healthy individuals (Merland et al., 1980; Thron, 2001). The lower thoracic region has relatively fewer venous outflow channels at a segmental level than the cervical or lumbosacral region (Tadie et al., 1985). These differences in segmental outflow probably contribute to the phenomenon that venous congestion is transmitted in a caudo-cranial direction throughout the spinal cord and that the first symptoms of myelopathy tend to reflect dysfunction of the lowest part of the cord, that is, the conus medullaris, even though the shunt is at the thoracic level, or in some cases even near the skull base (Vasdev et al., 1994; Asakawa et al., 2002). Venous outflow through the medullary vein and venous plexus is dorsal from the cord in 80–90%, and combined ventral and dorsal in 10–20% (Anson and Spetzler, 1993). In a post-mortem studies on thoracic radicular vasculature a physiological shunts between the radicular artery and a corresponding vein in subjects without previous symptoms of myelopathy is found (Fig. 4). The role of these shunts is unknown, and they have also been found in the lumbosacral spinal cord (Parke and Watanabe, 1985). Possibly arteriovenous shunts are not uncommon but they become symptomatic only through congenital or environmental factors that lead to impairment of venous outflow. Figure 4. The T7 segment of a 78-year-old woman. There is a shunt between the radicular artery and the venous plexus covering the outer surface of the dura (arrow).
- 13. Clinical features at onset The clinical features of SDAVF can be distinguished in those at the onset of the disease, in retrospect, and those present at the time of diagnosis. Initial symptoms are often non-specific. They include gait difficulties, symmetrical or asymmetrical sensory symptoms such as paraesthesias in one or both feet, diffuse or patchy sensory loss, but also radicular pain (Table 3). Disturbances of micturition and defaecation may occur at the start but most often they develop in later phases of the disease (Rosenblum et al., 1987; Lundqvist et al., 1990; Huffmann et al., 1995; Niimi et al., 1997; Westphal and Koch, 1999; Jellema et al., 2003; Koch et al., 2003). In the majority of patients (40–63%) progression lasts for 1–3 years before the diagnosis is made, but a protracted course with a duration of >3 years occurs in 10–34% (Logue, 1979; Symon et al., 1984; Koenig et al., 1989; Arnaud et al., 1994b; Lee et al., 1998; Sleiman et al., 1999; Van Dijk et al., 2002; Jellema et al., 2003). A gradually progressive course with stepwise deterioration is recorded in 11–32% of patients (Symon et al., 1984; Gilbertson et al., 1995; Jellema et al., 2003). Table 3. Proportion of patients with symptoms present at the onset of the disease and symptoms present at the time of diagnosis Initial symptoms (%) Symptoms at diagnosis (%) Sensory disturbances 17–72 63–100 Gait difficulties and motor disturbances 50–81 78–100 Pain (either pain in the back or radicular pain) 13–64 17–86 Micturition difficulties 4–75 62–91 Defaecation problems 0–38 30–100 Sexual dysfunction 0–17 11–80 An acute onset is reported in 5–18% of patients (Jellema et al., 2003). If symptoms develop within minutes to hours they can mimic an anterior spinal artery syndrome (Jellema et al., 2003). The sudden episodes mostly occur after exercise, prolonged standing and even singing (Khurana et al., 2002), and may disappear after rest. Acute worsening of symptoms may also be related to changes in posture such as bending over; even eating has been related with worsening of symptoms (Aminoff and Logue, 1974a; Symon et al., 1984; Rosenblum et al., 1987). Unusual presentations include an acute myelopathy after epidural anaesthesia (Sghirlanzoni et al., 1989) or development of an SDAVF after lumbar disc excision at the level of the left S1 root (Asakuno et al., 2002). A single case report described a 46-year-old woman who presented with intermittent episodes of weakness and sensory disturbances at the beginning of her menstrual periods (Kim et al., 1991). The explanation may be that uterine hyperaemia during the menstrual cycle increased the venous return in an already compromised pelvic venous system, which was indeed found at pelvic surgery. The patient was successfully treated with embolization of the fistula and removal of the uterus (Kim et al., 1991). In one patient an SDAVF was incidentally found 2 years before symptoms developed. He was treated after he eventually experienced progressive bilateral leg weakness and dysuria (Houdart et al., 2001). Intraspinal haemorrhage is distinctly rare, since it occurred in none of the 1178 patients described in the cumulative series with more than 5 patients (Table 2). A unique case report describes the only patient on record with an SDAVF at lumbar level (L4) who presented with spinal subarachnoid haemorrhage (Koch et al., 2004). Patients may present with acute headache as a result of intracranial subarachnoid headache (SAH), mostly in patients with a dural fistula at the craniocervical junction (Cahan et al., 1987; Willinsky et al., 1990; Morimoto et al., 1992; Ikeda et al., 1994; Kinouchi et al., 1998; Do et al., 1999; Hashimoto et al., 2000; Kim et al., 2003). Acute headache secondary to subarachnoid haemorrhage has been reported only twice in patients with a lower cervical SDAVF (Willinsky et al., 1990; Morimoto et al., 1992) and once in a patient with an SDAVF at T12 (Vates et al., 2001).
- 14. Clinical features at the time of diagnosis At the time of diagnosis there are often considerable neurological deficits (Table 3). At that stage two-thirds of patients show a combination of gait difficulties, sensory disturbances and involvement of sacral segments (micturition, defaecation or sexual dysfunction) (Jellema et al., 2003). Almost all patients by that time suffer from gait difficulties and weakness of one or both legs. Between 10–32% of patients are wheelchair-bound (Aminoff and Logue, 1974a; Rosenblum et al., 1987; Koenig et al., 1989; Mourier et al., 1989; Huffmann et al., 1995; Ushikoshi et al., 1999; Atkinson et al., 2001; Jellema et al., 2003; Koch et al., 2003). Upper motoneuron involvement (relatively increased tendon jerks or clonus, Babinski signs) and lower motoneuron involvement may be present at the same time, in agreement with the original observations of Foix and Alajouanine (Morgan and Marsh, 1989; Atkinson et al., 2001; Jellema et al., 2003). In one study, 69% of patients had both lower and upper motoneuron involvement (Atkinson et al., 2001). Upper motoneuron involvement may even be preceded by lower motoneuron involvement, other than in the two patients of Foix and Alajouanine; in a study that specifically addressed this question such a sequence was found in 11 of 33 patients (Jellema et al., 2003). Bowel and micturition problems frequently occur, mostly later in the course of the disease (Table 3). Micturition disturbances consist of urinary retention. Erectile dysfunction exists in 11–80% of men patients, and unwanted and involuntary ejaculations may occur after exercise (Jellema et al., 2003). A spontaneous and complete disappearance of the fistula has occasionally been described, though the clinical deficits remained unchanged in these patients (Renowden and Molyneux, 1993; Meder et al., 1995). Clinical diagnosis SDAVF is notoriously hard to diagnose, because of the misleading nature of the initial symptoms and the rarity of the disease. The median time between onset and diagnosis ranges between 12 and 44 months (Table 2). Since the first symptoms often consist of paraesthesiae and lower motor signs in the legs, this often suggests a neuromuscular disorder. Erroneous diagnoses often made initially are sensory polyneuropathy, acute or chronic inflammatory demyelinating polyneuropathy, spinal muscular atrophy and medullary tumour (Grandin et al., 1997; van der Meulen et al., 1999; Atkinson et al., 2001; Jellema et al., 2003). Not infrequently patients are unsuccessfully operated for a lumbar disc prolapse (Jellema et al., 2003). Although the clinical picture of a poly(radiculo)neuropathy may resemble that of SDAVF, especially in the early phase of the disease, there are some important differences. First, involvement of the arms is rare in SDAVF, and occurs only in cervical SDAVFs, whereas most polyneuropathies are eventually associated with a stocking-and-glove- like sensory loss. Secondly, though the sensory loss in SDAVF often begins distally, over time it extends more and more proximally, ultimately to the buttocks and the perineal region. Sensory involvement of the sacral dermatomes is exceptional in polyneuropathy. Thirdly, micturition problems are uncommon in polyneuropathies but occur eventually in almost 80% of patients with SDAVF (Symon et al., 1984; Rosenblum et al., 1987; Criscuolo et al., 1989; Huffmann et al., 1995; Linden and Berlit, 1996; Song et al., 2001; Van Dijk et al., 2002; Jellema et al., 2003). Mostly the disturbance consists of urinary retention, resulting from involvement of motor, sensory and autonomic neurons in the conus medullaris. Fourthly, asymmetrical sensory or motor deficits in the legs are a frequent feature in patients with SDAVF at the start of the disease, whereas in polyneuropathy symptoms are usually symmetrical. Fifthly and lastly, when upper motoneuron signs supervene (because the venous congestion in the spinal cord extends above the level of the conus medullaris) this is definitive proof that the lesion should not be localized in roots or nerves (Jellema et al., 2003). Investigations The essential investigations to establish the diagnosis are MRI and catheter angiography, which should be performed when a progressive myelopathy is suspected. MRI findings include hypo-intensities on T1-weighted images and hyper- intensities on T2-weighted images (Fig. 5). Increased signal intensity in the centre of the spinal cord and peripheral sparing on T2-weighted images is found in 67–100% of patients (Fig. 6) (Bowen et al., 1995; Gilbertson et al., 1995;
- 15. Jones et al., 1997; Hurst and Grossman, 2000; Luetmer et al., 2005). Hyperintensities extend over an average level of 5–7 vertebrae, with conus involvement over 80%, and are typically homogeneous (Gilbertson et al., 1995; Hurst and Grossman, 2000). Figure 5. MRI and angiography of SDAVF. (A) T2-weighted MRI image of a 66-year-old man with SDAVF. Multiple flow voids resembling an enlarged medullary draining vein can be seen. (B) Angiogram of a 57-year- old man showing an SDAVF at T7 on the right. Figure 6. Increased signal intensity in the centre of the spinal cord with peripheral sparing on a T2-weighted MRI image of a 60-year-old male with a left-sided SDAVF at T8. In addition, abnormalities suggesting abnormal blood vessels may be seen on either the ventral or the dorsal side of the spinal cord. These ‘flow void phenomena’ representing tortuous and dilated veins at the dorsal surface of the spinal cord are found in 35–91% of patients (Fig. 5) (Gilbertson et al., 1995; Hurst and Grossman, 2000). It seems that the flow voids are found more often, as studies are more recent, which may reflect advancement in MR techniques (Hurst and Grossman, 2000). The central hyperintense lesions are sometimes difficult to interpret, and may resemble anterior spinal artery infarction, myelitis or spinal cord neoplasms (Grandin et al., 1997), or, if slit-like, a persistent central canal (Holly and Batzdorf, 2002). Gadolinium-enhanced MRI scanning may reveal some contrast
- 16. enhancement of the spinal cord (Terwey et al., 1989). This is only minor immediately after injection of contrast agent, but marked enhancement occurs some 40–45 min after contrast injection (Terwey et al., 1989). MR angiography reveals flow in serpentine perimedullary structures in up to 100% of patients (Bowen et al., 1995; Mascalchi et al., 1995; Binkert et al., 1999; Mascalchi et al., 2001; Saraf-Lavi et al., 2002). MR angiography may also give an indication about the level of the SDAVF, which helps to confine the extent and duration of catheter angiography; the level of the fistula was correctly predicted in more than two-thirds of patients in several prospective series (Bowen et al., 1995; Mascalchi et al., 1999; Luetmer et al., 2005). Especially first-pass gadolinium-enhanced MR angiography results in correct prediction of the site of the fistula in nine patients with standard digital subtraction angiography used as gold standard (Farb et al., 2002). On the other hand, false-positive MR angiography is also possible in that normal vessels may be interpreted as being pathologically enlarged (Binkert et al., 1999; Luetmer et al., 2005). Sometimes multidetector row computed tomography is used in patients to find the site of the fistula (Bertrand et al., 2004; Lai et al., 2005). There is little experience with this technique in SDAVF, but it is evolving fast. Before the introduction of MRI, diagnosis was often made by means of myelography (N'Diaye et al., 1984; Gilbertson et al., 1995). This investigation would show an irregular, varicose dilation of the lumbar veins, giving the lumbar roots sometimes a ‘postage stamp’ appearance (with serrated edges) (N'Diaye et al., 1984). The enlarged vessels often extended over an average of 8 vertebrae (range: 3–20) (Gilbertson et al., 1995). Nowadays sometimes contrast- enhanced MR myelography is used (Chen and Hsu, 2002). In patients with a negative MRI scan (no swelling of the cord and hyperintensities on the T2-weighted images, no flow void phenomena ventral or dorsal to the surface of the spinal cord) and no findings resembling SDAVF on MR angiography, it is very unlikely that SDAVF is responsible for the symptoms (Bowen et al., 1995; Gilbertson et al., 1995; Saraf-Lavi et al., 2002). It seems justified to forgo catheter angiography in these patients. If there is still strong suspicion of an SDAVF, a myelogram can be performed (Gilbertson et al., 1995). This may show the above-mentioned features. A myelogram is also useful in patients in whom an SDAVF is suspected but in whom angiography is unsuccessful in detecting the fistula, for example because the orifice of lumbar arteries is obstructed by atherosclerosis (Oldfield et al., 2002). Catheter angiography is still the gold standard in the diagnosis of SDAVF (Fig. 5). Not only the intercostal and lumbar arteries should be visualized as potential feeding arteries of an abnormal shunt but also the median and lateral sacral artery, the deep cervical and ascending cervical arteries. If SDAVF is not found then intracranial vessels should be visualized, including the ascending pharyngeal artery, meningohypophyseal trunk, middle meningeal artery and occipital artery. The angioarchitecture of the fistula should be thoroughly investigated, especially with regard to the question whether the arterial feeder is a dural branch or a segmental medullary artery, which also contributes to the anterior spinal artery (Clavier et al., 1986). In the latter case endovascular treatment is not possible, because infarction of the spinal cord is likely to occur. Furthermore, it is essential to identify the artery of Adamkiewicz, because the fistula may originate from this important tributary to the anterior spinal artery (Aggarwal et al., 1992). In such patients, in whom the fistula originates from a common segmental artery, a radiopaque microcoil can be placed as a marker in the major feeding artery, which can then easily be visualized with conventional X-rays, allowing easy location of the fistula during a subsequent operation (Britz et al., 2004). Careful review of the angiographic images is essential. In a report about three patients who were strongly suspected of harbouring SDAVF on clinical and radiological grounds, no fistula could be demonstrated with angiography. They were nevertheless operated and the site of the fistula was identified and divided. In retrospect, in each case, the feeding vessel to the AVM had been visualized but not detected (Alleyne et al., 1999). Methods of treatment The choice of treatment is between endovascular embolization and surgical ligation of the fistula. Obviously the former option is the least invasive. Embolization with liquid polymers [such as isobutyl 2-cyanoacrylate (IBCA), n- butyl 2-cyanoacrylate (NBCA)] is advocated over particles such as polyvinyl alcohol (PVA), because the use of particles leads to a recurrence rate as high as 30–93% (Nichols et al., 1992). In contrast, occlusion is successful with
- 17. liquid polymers in 44–100% (Merland et al., 1986; Hall et al., 1989; Biondi et al., 1990; Niimi et al., 1997; Westphal and Koch, 1999; Van Dijk et al., 2002). The proportion of patients in whom recanalization occurs differs between studies, if only because of the different criteria that were used to define successful embolization. In one study, filling of the draining vein with glue was considered a successful embolization (Van Dijk et al., 2002). Other studies consider filling of the fistula itself as a measure of successful embolization (Niimi et al., 1997; Westphal and Koch, 1999). Embolization of SDAVF is not possible in every patient. First, if the arterial feeder of the fistula is a segmental medullary artery, embolization entails a high risk of spinal cord ischaemia. Secondly, technical difficulties such as arterial wall dissection of the feeding vessels during the embolization procedure may prohibit introduction of the microcatheter close enough to the fistula. If embolization is possible, then the success of treatment depends on endovascular occlusion of the draining vein, which means that recanalization will rarely occur when the draining vein is filled with glue (Fig. 7) (Jellema et al., 2005). The reason is probably that the fistula is often made up of several small feeding arteries and a single draining vein, so that occlusion of only (one of) the arterial feeder(s) to the fistula will generally lead to development of new arterial feeders (McCutcheon et al., 1996). Figure 7. Intradural localization of the glue cast, indicating that the draining vein is filled with glue. Surgical treatment before the pathophysiology of SDAVF was clear consisted of a multi-level laminectomy with stripping of the draining vein and decompression of the spinal cord (Krayenbuhl et al., 1969). Because it is now understood that with intradural fistulas the myelopathy is not caused by compression by the tangle of dilated veins on the dorsal surface of the cord, decompression is not indicated. Presently an intradural interruption of the vein draining the fistula is advocated (Huffmann et al., 1995). This is as effective as total removal of the draining vein (Afshar et al., 1995). A recent meta-analysis of patients with SDAVF who were treated with either embolization or operation showed that almost 98% of surgical procedures were technically successful; this stands in contrast to the 46% of patients who were successfully treated with embolization (Steinmetz et al., 2004). Outcome after treatment .The eventual outcome depends on several factors: duration of symptoms, pre-treatment disability and success of the
- 18. procedure to close the fistula. Treatment is directed at halting the progression of symptoms or even reversing them. Aminoff and Logue (1974b) were the first to describe the long-term follow-up of a group of patients. For this purpose they introduced the Aminoff–Logue disability scale (Table 4). A mean reduction of 1 grade on the Aminoff–Logue motor scale can be expected in a large proportion of patients (Song et al., 2001; Van Dijk et al., 2002; Cenzato et al., 2004; Jellema et al., 2004b). Table 4. Aminoff–Logue disability scales for gait and micturition (Aminoff and Logue, 1974b) Gait 0 Normal 1 Leg weakness, abnormal gait or stance, but no restriction of activity 2 Restricted activity but not requiring support 3 Requiring one stick for walking 4 Requiring two sticks, crutches or walker 5 Confined to wheelchair Micturition 0 Normal 1 Hesitancy, urgency, frequency, altered sensation, but continent 2 Occasional urinary incontinence or retention 3 Total incontinence or persistent retention A comparison between operative treatment and embolization in the long term is not possible, because of lack of longitudinal data for endovascular treatment (Steinmetz et al., 2004). In the review by these same authors improvement after operation was found in 55% of patients and worsening of symptoms in 11%. It seems plausible that patients with complete closure of the fistula by embolization (which is not always achieved; see previous section) show the same proportion of improvement, stabilization or worsening of symptoms as patients treated by operation. Recanalization will lead to neurological deterioration and requires additional treatment, through either endovascular intervention or operation. In some patients multiple endovascular interventions are needed to achieve permanent closure of the fistula (Jellema et al., 2004b, 2005). Symptoms that generally respond well to treatment are gait difficulties and muscle strength, resulting in less disability and dependence. In our own study of 44 patients who had been treated, mostly by embolization, after an average interval of almost 6 years, Jellema et al., 2004b found that walking disturbances improved in 64% of patients, and muscle strength in 56% (Jellema et al., 2004b). Others reported improvement in gait in 50–100% of patients (Niimi et al., 1997; Lee et al., 1998; Behrens and Thron, 1999; Ushikoshi et al., 1999; Westphal and Koch, 1999; Atkinson et al., 2001; Lev et al., 2001; Song et al., 2001; Cenzato et al., 2004; Guillevin et al., 2005). Micturition, pain and muscle spasms are symptoms that often respond less well to treatment than gait disability (Lundqvist et al., 1990; Song et al., 2001; Eskandar et al., 2002; Jellema et al., 2004b; Guillevin et al., 2005), but one study nevertheless reported marked improvement of micturition and bowel function (Van Dijk et al., 2002). No reliable prognostic factors have been identified. A relatively short delay in diagnosis predicted better outcome in one study (Niimi et al., 1997), but not in others (Eskandar et al., 2002; Cenzato et al., 2004; Jellema et al., 2004b). One might expect that pretreatment disability rather than the length of the interval before diagnosis is a good measure of the eventual outcome, but this was confirmed in only a few studies (Eskandar et al., 2002; Cenzato et al., 2004), and not in another (Jellema et al., 2004b). The location of the fistula was a prognostic factor in one study (Cenzato et al., 2004). In this Italian series patients
- 19. with a fistula located between T9 and T12 responded better to treatment than those with a fistula elsewhere. The authors explained this by the better vascularization of the lower thoracic cord than that of the upper thoracic cord. Future studies on long-term follow-up should include a complete neurological examination in a large group of patients to determine prognostic factors. Outcome measures should reflect disability and handicap rather than neurological deficits, should not be disease-specific but generic and should be sufficiently established to be used for comparing separate studies. The modified Rankin scale, alternatively called the Oxford handicap scale, is a good example (Bamford et al., 1989). In conclusion, SDAVF is an intriguing and enigmatic disease entity, which is only slowly relinquishing its secrets. The pathophysiology is now well understood, much better than the causes for the development of the fistulas. Because the disease is rare, the knowledge about this disease is progressing at a slow pace. Yet the knowledge about the condition deserves to be wide. Every neurologist should be aware of this potential curable disease and should be able to diagnose this disease at an early stage, to prevent the impending disaster of paraplegia. In The Netherlands, there are 600 neurologists in a population of 16 million. With an estimated incidence of 5–10/million/year (Thron, 2001), a neurologist will see about one patient with SDAVF in 4–8 years. Despite the low frequency at which a neurologist will encounter a patient with SDAVF, continuous education is imperative to ensure a high degree of awareness to diagnose this condition. SUMMARY SUMMARY Spinal Dural AVFs, most common type of spinal vascular malformation constituting 80% of spinal AVMs. There are three basic types of AVFs - extradural, dorsal intradural, and ventral intradural. Dorsal intradural AVFs have been given a number of alternate names including long dorsal, angioma racemosum, dorsal extramedullary, angioma racemosum venosum, and Type I. The current literature supports use of term Type I. Type I is further classified into subtypes A and B to designate AVMs with single or multiple feeding arteries, respectively. These dural AVFs are malformations that most commonly occur in the thoracic region. The dural AVFs consist of an abnormal communication between a radicular artery and a radicular vein within the dural sleeve of a nerve root [1]. The vein communicates with the coronal venous plexus along the surface of the spinal cord. The clinical manifestation of patients with this miscommunication is the insidious development of venous hypertension induced-progressive myelopathy. Venous congestion produces progressive neurological deterioration that can manifest as sensory disturbances, paraparesis, and sphincter dysfunction. The pathognomonic MR imaging studies is a pencil-like hyperintensity in the spinal cord on a T2-weighted image. Within three years of developing impairment the patients became severely disabled. This and similar outcomes has led to the rule that elimination of dural AVFs will halt the progressive neurological deterioration. MR imaging findings of Type I AVMs include enlargement of the spinal cord, cord hypointensity on Tl-weighted images, central cord hyperintensity on T2-weighted images, scalloping of the surface of the cord on sagittal images, serpentine flow voids, and enhancement of the cord and dilated perimedullary veins after administration of gadopentetate dimeglumine. Hyperintensity of the cord on T2-weighted images is believed to be the most sensitive finding. Increased T2 signal intensity is thought to result from cord edema. Although high signal intensity on T2-weighted images is the most sensitive sign, it is nonspecific and can be seen in inflammatory, neoplastic, degenerative, demyelinating, and posttraumatic conditions.
- 20. The eliminations of dural AVFs can be performed through endovascular or surgical therapy. Endovascular therapy consists of embolization with liquid acrylic embolic material into the AVF and/or the proximal draining vein. Surgical therapy involves microsurgical ligation of AVF draining vein and excision of affected dura1. Addendum A new version of this PDF file (with a new case) is uploaded in my web site every week (every Saturday and remains available till Friday.) To download the current version follow the link "http://pdf.yassermetwally.com/case.pdf". You can also download the current version from my web site at "http://yassermetwally.com". To download the software version of the publication (crow.exe) follow the link: http://neurology.yassermetwally.com/crow.zip The case is also presented as a short case in PDF format, to download the short case follow the link: http://pdf.yassermetwally.com/short.pdf At the end of each year, all the publications are compiled on a single CD-ROM, please contact the author to know more details. Screen resolution is better set at 1024*768 pixel screen area for optimum display. For an archive of the previously reported cases go to www.yassermetwally.net, then under pages in the right panel, scroll down and click on the text entry "downloadable case records in PDF format" Also to view a list of the previously published case records follow the following link (http://wordpress.com/tag/case-record/) or click on it if it appears as a link in your PDF reader REFERENCES References 1. Afshar JK, Doppman JL, Oldfield EH. (1995) Surgical interruption of intradural draining vein as curative treatment of spinal dural arteriovenous fistulas. J Neurosurg 82:196–200. 2. Aggarwal S, Willinsky R, Montanera W, terBrugge K, Wallace MC. (1992) Superselective angiography of a spinal dural arteriovenous fistula having a common segmental origin with the artery of Adamkiewicz. Neuroradiology 34:352–4. 3. Alleyne CHJ, Barrow DL, Joseph G. (1999) Surgical management of angiographically occult spinal dural arteriovenous fistulae (type I spinal arteriovenous malformations): three technical case reports. Neurosurgery 44:891–4. 4. Aminoff MJ. (1976) Spinal angiomas. (Blackwell Scientific Publications, Oxford). 5. Aminoff MJ and Logue V. (1974a) Clinical features of spinal vascular malformations. Brain 97:197–210. 6. Aminoff MJ and Logue V. (1974b) The prognosis of patients with spinal vascular malformations. Brain 97:211– 8. 7. Aminoff MJ, Barnard RO, Logue V. (1974) The pathophysiology of spinal vascular malformations. J Neurol Sci 23:255–63. 8. Anson JA and Spetzler RF. (1993) Spinal dural arteriovenous malformations. In Award IA and Barrow DL (Eds.). Dural arteriovenous malformations(American Association of Neurological Surgeons Publications Committee, Park Ridge, IL) pp. 175–91.
- 21. 9. Antoni N. (1962) Spinal vascular malformation (angiomas) and myelomalcie. Neurology 12:795–804. 10. Arnaud O, Bille F, Pouget J, Serratrice G, Salamon G. (1994a) Epidural arteriovenous fistula with perimedullary venous drainage: case report. Neuroradiology 36:490–1. 11. Arnaud O, Pelletier J, Dalecky A, Cherif AA, Azulay JP, Salamon G, et al. (1994b) [Spinal dural fistula with peri-medullar venous drainage]. Rev Neurol (Paris) 150:713–20. 12. Asakawa H, Yanaka K, Fujita K, Marushima A, Anno I, Nose T. (2002) Intracranial dural arteriovenous fistula showing diffuse MR enhancement of the spinal cord: case report and review of the literature. Surg Neurol 58:251–7. 13. Asakuno K, Kim P, Kawamoto T, Ogino M. (2002) Dural arteriovenous fistula and progressive conus medullaris syndrome as complications of lumbar discectomy. Case report. J Neurosurg 97:375–9. 14. Atkinson JLD, Miller GM, Krauss WE, Marsh WR, Piepgras DG, Atkinson PP, et al. (2001) Clinical and radiographic features of dural arteriovenous fistula, a treatable cause of myelopathy. Mayo Clin Proc 76:1120– 30. 15. Bamford JM, Sandercock PA, Warlow CP, Slattery J. (1989) Interobserver agreement for the assessment of handicap in stroke patients. Stroke 20:828. 16. Barnwell SL, Halbach VV, Dowd CF, Higashida RT, Hieshima GB, Wilson CB. (1991) Multiple dural arteriovenous fistulas of the cranium and spine. AJNR Am J Neuroradiol 12:441–5. 17. Barrow DL. (2002) Spinal cord vascular lesions. J Neurosurg 96:143–4. 18. Barrow DL, Colohan AR, Dawson R. (1994) Intradural perimedullary arteriovenous fistulas (type IV spinal cord arteriovenous malformations). J Neurosurg 81:221–9. 19. Bedersen JB and Spetzler RF. (1996) Pathophysiology of Type I spinal dural arteriovenous malformations. BNI Quarterly 12:23–32. 20. Behrens S and Thron A. (1999) Long-term follow-up and outcome in patients treated for spinal dural arteriovenous fistula. J Neurol 246:181–5. 21. Berenstein A and Lasjaunias P. (1992) Surgical neuro-angiography: endovascular treatment of spine and spinal cord lesions. (Springer-Verlag, Berlin). 22. Berenstein A, Lasjaunias P, Ter Brugge KG. (2004) Surgical neuroangiography 2.2. (Springer, Berlin). 23. Bertrand D, Douvrin F, Gerardin E, Clavier E, Proust F, Thiebot J. (2004) Diagnosis of spinal dural arteriovenous fistula with multidetector row computed tomography: a case report. Neuroradiology 46:851–4. 24. Binkert CA, Kollias SS, Valavanis A. (1999) Spinal cord vascular disease: characterization with fast three- dimensional contrast-enhanced MR angiography. AJNR Am J Neuroradiol 20:1785–93. 25. Biondi A, Merland JJ, Reizine D, Aymard A, Hodes JE, Lecoz P, et al. (1990) Embolization with particles in thoracic intramedullary arteriovenous malformations: long-term angiographic and clinical results. Radiology 177:651–8. 26. Borden JA, Wu JK, Shucart WA. (1995) A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg 82:166–79. 27. Bowen BC, Fraser K, Kochan JP, Pattany PM, Green BA, Quencer RM. (1995) Spinal dural arteriovenous
- 22. fistulas: evaluation with MR angiography. AJNR Am J Neuroradiol 16:2029–43. 28. Eur Neurol Bradac GB, Daniele D, Riva A, Bracchi M, Stura G, Riccio A, et al. (1994) Spinal dural arteriovenous fistulas: an underestimated cause of myelopathy. 34:87–94. 29. Brion S, Netzky MG, Zimmerman HM. (1952) Vascular malformations of the spinal cord. AMA Arch Neurol Psychiatry 68:339–61. 30. Britz GW, Lazar D, Eskridge J, Winn HR. (2004) Accurate intraoperative localization of spinal dural arteriovenous fistulae with embolization coil: technical note. Neurosurgery 55:252–4. 31. Brunereau L, Gobin YP, Meder JF, Cognard C, Tubiana JM, Merland JJ. (1996) Intracranial dural arteriovenous fistulas with spinal venous drainage: relation between clinical presentation and angiographic findings. AJNR Am J Neuroradiol 17:1549–54. 32. Cahan LD, Higashida RT, Halbach VV, Hieshima GB. (1987) Variants of radiculomeningeal vascular malformations of the spine. J Neurosurg 66:333–7. 33. Cenzato M, Versari P, Righi C, Simionato F, Casali C, Giovanelli M. (2004) Spinal dural arteriovenous fistulae: analysis of outcome in relation to pretreatment indicators. Neurosurgery 55:815–22. 34. Chaloupka JC, Gobin YP, Guglielmi G, Steichen JD, Vinuela F. (1995) Two concurrent spinal dural arteriovenous fistulae in a patient with rapidly progressive myelopathy. A case report. Angiology 46:251–7. 35. Chen CJ and Hsu HL. (2002) Engorged and tortuous intradural filum terminale vein as a sign of a sacral dural arteriovenous malformation. Eur J Radiol 44:152–5. 36. Choi IS. (1992) Spinal dural arteriovenous fistula: the role of PVA embolization. AJNR Am J Neuroradiol 13:941–2. 37. Clavier E, Tadie M, Thiebot J, Presles O, Benozio M. (1986) Common origin of the arterial blood flow for an arteriovenous medullar fistula and the anterior spinal artery: a case report. Neurosurgery 18:660–3. 38. Coats TJ and King TT. (1991) The diagnosis of dural spinal vascular malformations. Br J Neurosurg 5:609–15. 39. Criscuolo GR, Oldfield EH, Doppman JL. (1989) Reversible acute and subacute myelopathy in patients with dural arteriovenous fistulas. Foix–Alajouanine syndrome reconsidered. J Neurosurg 70:354–9. 40. Djindjian R. (1972) Neurological examination of spinal cord angiomas. In Vinken PJ and Bruyn GW (Eds.). Handbook of clinical neurology(Elsevier, Amsterdam) pp. 631–43. 41. Djindjian R. (1976) Spinal vascular malformations. J Neurosurg 45:727–8. 42. Djindjian M, Djindjian R, Rey A, Hurth M, Houdart R. (1977) Intradural extramedullary spinal arterio-venous malformations fed by the anterior spinal artery. Surg Neurol 8:85–93. 43. Do HM, Jensen ME, Cloft HJ, Kallmes DF, Dion JE. (1999) Dural arteriovenous fistula of the cervical spine presenting with subarachnoid hemorrhage. AJNR Am J Neuroradiol 20:348–50. 44. Doppman JL, Di Chiro G, Ommaya A. (1968) Obliteration of spinal-cord arteriovenous malformation by percutaneous embolisation. Lancet 1:477. 45. Elsberg CA. (1916) Diagnosis and treatment of surgical diseases of the spinal cord and its membranes. (W.B. Saunders, Philadelphia, PA)194–204.
- 23. 46. Eskandar EN, Borges LF, Budzik RFJ, Putman CM, Ogilvy CS. (2002) Spinal dural arteriovenous fistulas: experience with endovascular and surgical therapy. J Neurosurg 96:162–7. 47. Farb RI, Kim JK, Willinsky RA, Montanera WJ, terBrugge K, Derbyshire JA, et al. (2002) Spinal dural arteriovenous fistula localization with a technique of first-pass gadolinium-enhanced MR angiography: initial experience. Radiology 222:843–50. 48. Foix CH and Alajouanine Th. (1926) La myélite nécrotique subaigüe. Rev Neurol 46:1–42. 49. Gilbertson JR, Miller GM, Goldman MS, Marsh WR. (1995) Spinal dural arteriovenous fistulas: MR and myelographic findings. AJNR Am J Neuroradiol 16:2049–57. 50. Goyal M, Willinsky R, Montanera W, terBrugge K. (1999) Paravertebral arteriovenous malformations with epidural drainage: clinical spectrum, imaging features, and results of treatment. AJNR Am J Neuroradiol 20:749–55. 51. Grandin C, Duprez T, Stroobandt G, Laterre EC, Mathurin P. (1997) Spinal dural arterio-venous fistula: an underdiagnosed disease? Acta Neurol Belg 97:17–21. 52. Guillevin R, Vallee JN, Cormier E, Lo D, Dormont D, Chiras J. (2005) N-butyl 2-cyanoacrylate embolization of spinal dural arteriovenous fistulae: CT evaluation, technical features, and outcome prognosis in 26 cases. AJNR Am J Neuroradiol 26:929–35. 53. Hall WA, Oldfield EH, Doppman JL. (1989) Recanalization of spinal arteriovenous malformations following embolization. J Neurosurg 70:714–20. 54. Hashimoto H, Iida J, Shin Y, Hironaka Y, Sakaki T. (2000) Spinal dural arteriovenous fistula with perimesencephalic subarachnoid haemorrhage. J Clin Neurosci 7:64–6. 55. Hassler W, Thron A, Grote EH. (1989) Hemodynamics of spinal dural arteriovenous fistulas. An intraoperative study. J Neurosurg 70:360–70. 56. Hassler W and Thron A. (1994) Flow velocity and pressure measurements in spinal dural arteriovenous fistulas. Neurosurg Rev 17:29–36. 57. Heros RC, Debrun GM, Ojemann RG, Lasjaunias PL, Naessens PJ. (1986) Direct spinal arteriovenous fistula: a new type of spinal AVM. Case report. J Neurosurg 64:134–9. 58. Hoffman HL. (1955) Acute necrotic myelopathy. Brain 78:377–94. 59. Holly LT and Batzdorf U. (2002) Slitlike syrinx cavities: a persistent central canal. J Neurosurg 97:161–5. 60. Houdart R, Djindjian R, Hurth M. (1966) Vascular malformations of the spinal cord. The anatomic and therapeutic significance of arteriography. J Neurosurg 24:583–94. 61. Houdart E, Redondo A, Saint-Maurice JP, Merland JJ. (2001) Natural history of an incidentally discovered spinal dural arteriovenous fistula. Neurology 57:742–3. 62. Huffmann BC, Gilsbach JM, Thron A. (1995) Spinal dural arteriovenous fistulas: a plea for neurosurgical treatment. Acta Neurochir (Wien) 135:44–51. 63. Hurst RW and Grossman RI. (2000) Peripheral spinal cord hypointensity on T2-weighted MR images: a reliable imaging sign of venous hypertensive myelopathy. AJNR Am J Neuroradiol 21:781–6. 64. Hurst RW, Kenyon LC, Lavi E, Raps EC, Marcotte P. (1995) Spinal dural arteriovenous fistula: the pathology
- 24. of venous hypertensive myelopathy. Neurology 45:1309–13. 65. Ikeda H, Fujimoto Y, Koyama T, Fujimoto Y. (1994) [A rare case of high cervical spinal cord dural arteriovenous fistula presenting with intracranial subarachnoid hemorrhage]. No Shinkei Geka 22:1045–8. 66. Jellema K, Canta LR, Tijssen CC, van Rooij WJ, Koudstaal PJ, van Gijn J. (2003) Spinal dural arteriovenous fistulas: clinical features in 80 patients. J Neurol Neurosurg Psychiatry 74:1438–40. 67. Jellema K, Tijssen CC, Fijnheer R, De Groot PG, Koudstaal PJ, van Gijn J. (2004a) Spinal dural arteriovenous fistulas are not associated with prothrombotic factors. Stroke 35:2069–71. 68. Jellema K, Tijssen CC, van Rooij WJ, Sluzewski M, Koudstall PJ, Algra A, et al. (2004b) Spinal dural arteriovenous fistulas: long-term follow-up of 44 treated patients. Neurology 62:1839–41. 69. Jellema K, Sluzewski M, van Rooij WJ, Tijssen CC, Beute GN. (2005) Embolization of spinal dural arteriovenous fistulas: importance of occlusion of the draining vein. J Neurosurg Spine 2:580–3. 70. Jellema K, Tijssen CC, Sluzewski M, van Asbeck FW, Koudstaal PJ, van Gijn J. (2006) Spinal dural arteriovenous fistulas—an underdiagnosed disease A review of patients admitted to the spinal unit of a rehabilitation center. J Neurol 253: pp. 159–62. 71. Jellinger K, Minauf M, Garzuly F, Neumayer E. (1968) [Angiodysgenetic necrotizing myelopathy (report on 7 cases)]. Arch Psychiatr Nervenkr 211:377–404. 72. Jones BV, Ernst RJ, Tomsick TA, Tew JJ. (1997) Spinal dural arteriovenous fistulas: recognizing the spectrum of magnetic resonance imaging findings. J Spinal Cord Med 20:43–8. 73. Kataoka H, Miyamoto S, Nagata I, Ueno Y, Hashimoto N. (1999) Intraoperative microdoppler monitoring for spinal dural arteriovenous fistulae. Surg Neurol 52:466–72. 74. Kataoka H, Miyamoto S, Nagata I, Ueba T, Hashimoto N. (2001) Venous congestion is a major cause of neurological deterioration in spinal arteriovenous malformations. Neurosurgery 48:1224–9. 75. Katz JD and Ropper AH. (2000) Progressive necrotic myelopathy: clinical course in 9 patients. Arch Neurol 57:355–61. 76. Kendall BE and Logue V. (1977) Spinal epidural angiomatous malformations draining into intrathecal veins. Neuroradiology 13:181–9. 77. Khurana VG, Perez-Terzic CM, Petersen RC, Krauss WE. (2002) Singing paraplegia: a distinctive manifestation of a spinal dural arteriovenous fistula. Neurology 58:1279–81. 78. Kim DI, Choi IS, Berenstein A. (1991) A sacral dural arteriovenous fistula presenting with an intermittent myelopathy aggravated by menstruation. Case report. J Neurosurg 75:947–9. 79. Kim MS, Han DH, Han MH, Oh CW. (2003) Posterior fossa hemorrhage caused by dural arteriovenous fistula: case reports. Surg Neurol 59:512–6. 80. Kinouchi H, Mizoi K, Takahashi A, Nagamine Y, Koshu K, Yoshimoto T. (1998) Dural arteriovenous shunts at the craniocervical junction. J Neurosurg 89:755–61. 81. Koch C, Kucinski T, Eckert B, Rother J, Zeumer H. (2003) [Spinal dural arteriovenous fistula: clinical and radiological findings in 54 patients]. Rofo 175:1071–8. 82. Koch C, Gottschalk S, Giese A. (2004) Dural arteriovenous fistula of the lumbar spine presenting with
- 25. subarachnoid hemorrhage. Case report and review of the literature. J Neurosurg 100:385–91. 83. Koenig E, Thron A, Schrader V, Dichgans J. (1989) Spinal arteriovenous malformations and fistulae: clinical, neuroradiological and neurophysiological findings. J Neurol 236:260–6. 84. Kraus JA, Stuper BK, Berlit P. (1998) Association of resistance to activated protein C and dural arteriovenous fistulas. J Neurol 245:731–3. 85. Kraus JA, Stuper BK, Nahser HC, Klockgether T, Berlit P. (2000) Significantly increased prevalence of factor V Leiden in patients with dural arteriovenous fistulas. J Neurol 247:521–3. 86. Krause F. (1911) Chirurgie des Gehirns und Ruckenmarks nach eigenen Erfarungen. (Urban & Schwarzenberg, Berlin). 87. Krayenbuhl H, Yasargil MG, McClintock HG. (1969) Treatment of spinal cord vascular malformations by surgical excision. J Neurosurg 30:427–35. 88. Krings T, Mull M, Reinges MH, Thron A. (2004) Double spinal dural arteriovenous fistulas: case report and review of the literature. Neuroradiology 46:238–42. 89. Lai PH, Pan HB, Yang CF, Yeh LR, Hsu SS, Lee KW, et al. (2005) Multi-detector row computed tomography angiography in diagnosing spinal dural arteriovenous fistula: initial experience. Stroke 36:1562–4. 90. Lee TT, Gromelski EB, Bowen BC, Green BA. (1998) Diagnostic and surgical management of spinal dural arteriovenous fistulas. Neurosurgery 43:242–6. 91. Lev N, Maimon S, Rappaport ZH, Melamed E. (2001) Spinal dural arteriovenous fistulae—a diagnostic challenge. Isr Med Assoc J 3:492–6. 92. Lhermitte J, Fribourg-Blanc J, Kyriaco N. (1931) La gliose angéio-hypertrophique de la moelle épinière (myélite nécrotique de Foix-Alajouanine). Rev Neurol 2:37–52. 93. Li M, Zhang HQ, Zhi XL, Zhang P, Ling F. (2005) Surgical interruption of spinal dural arteriovenous fistulas. Chin Med J (Engl) 118:433–5. 94. Linden D and Berlit P. (1996) Spinal arteriovenous malformations: clinical and neurophysiological findings. J Neurol 243:9–12. 95. Logue V. (1979) Angiomas of the spinal cord: review of the pathogenesis, clinical features, and results of surgery. J Neurol Neurosurg Psychiatry 42:1–11. 96. Luetmer PH, Lane JI, Gilbertson JR, Bernstein MA, Huston J III, Atkinson JL. (2005) Preangiographic evaluation of spinal dural arteriovenous fistulas with elliptic centric contrast-enhanced MR angiography and effect on radiation dose and volume of iodinated contrast material. AJNR Am J Neuroradiol 26:711–8. 97. Lundqvist C, Berthelsen B, Sullivan M, Svendsen P, Andersen O. (1990) Spinal arteriovenous malformations: neurological aspects and results of embolization. Acta Neurol Scand 82:51–8. 98. Malis LI. (1982) Neurological surgery: a comprehensive reference guide to the diagnosis and management of neurosurgical problems. (W.B. Saunders, Philadelphia). 99. Mascalchi M, Bianchi MC, Quilici N, Mangiafico S, Ferrito G, Padolecchia R, et al. (1995) MR angiography of spinal vascular malformations. AJNR Am J Neuroradiol 16:289–97. 100. Mascalchi M, Cosottini M, Ferrito G, Quilici N, Bartolozzi C, Villari N. (1999) Contrast-enhanced time-resolved
- 26. MR angiography of spinal vascular malformations. J Comput Assist Tomogr 23:341–5. 101. Mascalchi M, Ferrito G, Quilici N, Mangiafico S, Cosottini M, Cellerini M, et al. (2001) Spinal vascular malformations: MR angiography after treatment. Radiology 219:346–53. 102. McCutcheon IE, Doppman JL, Oldfield EH. (1996) Microvascular anatomy of dural arteriovenous abnormalities of the spine: a microangiographic study. J Neurosurg 84:215–20. 103. Meder JF, Devaux B, Merland JJ, Fredy D. (1995) Spontaneous disappearance of a spinal dural arteriovenous fistula. AJNR Am J Neuroradiol 16:2058–62. 104. Merland JJ, Riche MC, Chiras J. (1980) Intraspinal extramedullary arteriovenous fistulae draining into the medullary veins. J Neuroradiol 7:271–320. 105. Merland JJ, Assouline E, Rufenacht D, Guimaraens L, Laurent A. (1986) Dural spinal arteriovenous fistulae draining into medullary veins: clinical and radiological results of treatment (embolization and surgery) in 56 cases. Excerpta Med Int Congr Ser 698:283–9. 106. Morgan MK and Marsh WR. (1989) Management of spinal dural arteriovenous malformations. J Neurosurg 70:832–6. 107. Morimoto T, Yoshida S, Basugi N. (1992) Dural arteriovenous malformation in the cervical spine presenting with subarachnoid hemorrhage: case report. Neurosurgery 31:118–20. 108. Mourier KL, Gelbert F, Rey A, Assouline E, George B, Reizine D, et al. (1989) Spinal dural arteriovenous malformations with perimedullary drainage. Indications and results of surgery in 30 cases. Acta Neurochir (Wien) 100:136–41. 109. N'Diaye M, Chiras J, Meder JF, Barth MO, Koussa A, Bories J. (1984) Water-soluble myelography for the study of dural arteriovenous fistulae of the spine draining in the spinal venous system. J Neuroradiol 11:327–39. 110. Nichols DA, Rufenacht DA, Jack CRJ, Forbes GS. (1992) Embolization of spinal dural arteriovenous fistula with polyvinyl alcohol particles: experience in 14 patients. AJNR Am J Neuroradiol 13:933–40. 111. Niimi Y, Berenstein A, Setton A, Neophytides A. (1997) Embolization of spinal dural arteriovenous fistulae: results and follow-up. Neurosurgery 40:675–82. 112. Oldfield EH and Doppman JL. (1988) Spinal arteriovenous malformations. Clin Neurosurg 34:161–83. 113. Oldfield EH, Di Chiro G, Quindlen EA, Rieth KG, Doppman JL. (1983) Successful treatment of a group of spinal cord arteriovenous malformations by interruption of dural fistula. J Neurosurg 59:1019–30. 114. Oldfield EH, Bennett A, Chen MY, Doppman JL. (2002) Successful management of spinal dural arteriovenous fistulas undetected by arteriography. Report of three cases. J Neurosurg 96:220–9. 115. Parke WW and Watanabe R. (1985) The intrinsic vasculature of the lumbosacral spinal nerve roots. Spine 10:508–15. 116. Pia HW and Vogelsang H. (1965) [Diagnosis and therapy of spinal angioma.]. Dtsch Z Nervenheilkd 187:74–96. 117. Pierot L, Vlachopoulos T, Attal N, Martin N, Bert S, Chiras J. (1993) Double spinal dural arteriovenous fistulas: report of two cases. AJNR Am J Neuroradiol 14:1109–12. 118. Renowden SA and Molyneux AJ. (1993) Case report: spontaneous thrombosis of a spinal dural AVM (Foix– Alajouanine syndrome)—magnetic resonance appearance. Clin Radiol 47:134–6.
- 27. 119. Rosenblum B, Oldfield EH, Doppman JL, Di Chiro G. (1987) Spinal arteriovenous malformations: a comparison of dural arteriovenous fistulas and intradural AVM's in 81 patients. J Neurosurg 67:795–802. 120. Saraf-Lavi E, Bowen BC, Quencer RM, et al. (2002) Detection of spinal dural arteriovenous fistulae with MR imaging and contrast-enhanced MR angiography: sensitivity, specificity, and prediction of vertebral level. AJNR Am J Neuroradiol 23:858–67. 121. Schick U and Hassler W. (2003) Treatment and outcome of spinal dural arteriovenous fistulas. Eur Spine J 12:350–5. 122. Scholz W and Wechsler W. (1959) [A further contribution to angiodysgenetic necrotizing myelopathy (Foix– Alajouanine disease).]. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr 199:609–29. 123. Sghirlanzoni A, Gemma M, Pareyson D, Cimino C, Boiardi A. (1989) Spinal arteriovenous fistula. A possible cause of paraparesis after epidural anaesthesia. Anaesthesia 44:831–3. 124. Sleiman M, Leclerc X, Lejeune JP, Pruvo JP, Duquesnoy B, Christiaens JL. (1999) [Clinical aspects and treatment of dural-spinal arteriovenous fistulas with perimedullary venous drainage. 10 cases.]. Neurochirurgie 45:276–85. 125. Song JK, Vinuela F, Gobin YP, Song JK, Duckwiler GR, Murayama Y, et al. (2001) Surgical and endovascular treatment of spinal dural arteriovenous fistulas: long-term disability assessment and prognostic factors. J Neurosurg 94:199–204. 126. Spetzler R, Detwiler P, Riina H, Porter R. (2002) Modified classification of spinal vascular lesions. J Neurosurg 96:145–56. 127. Steinmetz MP, Chow MM, Krishnaney AA, Andrews-Hinders D, Benzel EC, Masaryk TJ, et al. (2004) Outcome after the treatment of spinal dural arteriovenous fistulae: a contemporary single-institution series and meta- analysis. Neurosurgery 55:77–87. 128. Symon L, Kuyama H, Kendall B. (1984) Dural arteriovenous malformations of the spine. Clinical features and surgical results in 55 cases. J Neurosurg 60:238–47. 129. Tacconi L, Lopez Izquierdo BC, Symon L. (1997) Outcome and prognostic factors in the surgical treatment of spinal dural arteriovenous fistulas. A long-term study. Br J Neurosurg 11:298–305. 130. Tadie M, Hemet J, Freger P, Clavier E, Creissard P. (1985) Morphological and functional anatomy of spinal cord veins. J Neuroradiol 12:3–20. 131. Terwey B, Becker H, Thron AK, Vahldiek G. (1989) Gadolinium-DTPA enhanced MR imaging of spinal dural arteriovenous fistulas. J Comput Assist Tomogr 13:30–7. 132. Thron A. (2001) [Spinal dural arteriovenous fistulas.]. Radiologe 41:955–60. 133. Tsai LK, Jeng JS, Liu HM, Wang HJ, Yip PK. (2004) Intracranial dural arteriovenous fistulas with or without cerebral sinus thrombosis: analysis of 69 patients. J Neurol Neurosurg Psychiatry 75:1639–41. 134. Ushikoshi S, Hida K, Kikuchi Y, Miyasaka K, Iwasaki T, Abe H. (1999) Functional prognosis after treatment of spinal dural arteriovenous fistulas. Neurol Med Chir (Tokyo) 39:206–12. 135. van der Meulen MF, Rinkel GJ, Witkamp TD, van Gijn J. (1999) A man with progressive weakness in his legs. Lancet 354:830. 136. Van Dijk JM, TerBrugge KG, Willinsky RA, Farb RI, Wallace MC. (2002) Multidisciplinary management of
- 28. spinal dural arteriovenous fistulas: clinical presentation and long-term follow-up in 49 patients. Stroke 33:1578– 83. 137. Vasdev A, Lefournier V, Bessou P, Dematteis M, Crouzet G. (1994) [Intracranial dural fistula with spinal cord venous drainage. Apropos of 2 cases.]. J Neuroradiol 21:134–54. 138. Vates GE, Quinones-Hinojosa A, Halbach VV, Lawton MT. (2001) Conus perimedullary arteriovenous fistula with intracranial drainage: case report. Neurosurgery 49:457–61. 139. Westphal M and Koch C. (1999) Management of spinal dural arteriovenous fistulae using an interdisciplinary neuroradiological/neurosurgical approach: experience with 47 cases. Neurosurgery 45:451–7. 140. Willinsky R, terBrugge K, Lasjaunias P, Montanera W. (1990) The variable presentations of craniocervical and cervical dural arteriovenous malformations. Surg Neurol 34:118–23. 141. Wyburn-Mason R. (1943) The vascular abnormalities and tumours of the spinal cord and its membranes. (Henry Kimpton, London). 142. Metwally, MYM: Textbook of neuroimaging, A CD-ROM publication, (Metwally, MYM editor) WEB-CD agency for electronic publication, version 10.4a October 2009
