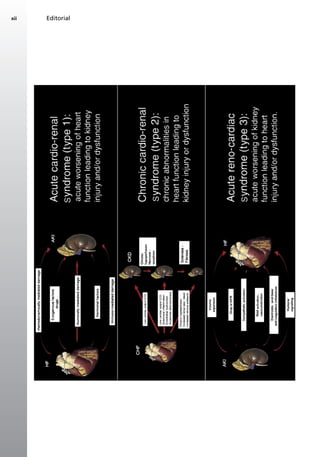
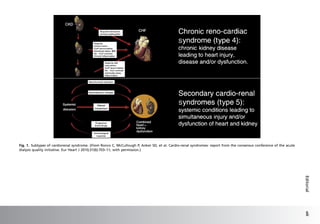
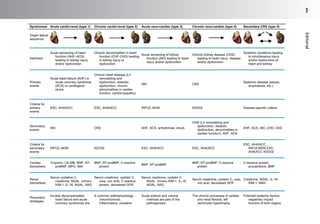
This document discusses anemia in patients with heart failure and kidney disease (cardiorenal anemia). It notes that anemia is common in these patients and associated with higher mortality and costs. The pathophysiology of cardiorenal disease and anemia are both multifactorial, involving interactions between cardiac function, renal dysfunction, inflammation, and other factors. The document outlines five subtypes of cardiorenal syndrome based on whether heart or kidney dysfunction is acute or chronic. It emphasizes that a multidisciplinary approach is needed to manage cardiorenal anemia given its complex causes.


![responses, hemodilution, iron deficiency, impaired
ability to use available iron stores,10
bone marrow
suppression due to cytokines (eg, tumor necrosis
factor a [TNF-a], interleukin [IL]-1, IL-6, and
C-reactive protein), blunted bone marrow respon-
siveness to erythropoietin, impaired iron mobiliza-
tion, and effects of medications. Aspirin and
angiotensin-converting enzyme inhibitors11
contribute to the anemia potentially through the
actions of hematopoesis inhibitor, N-acetyl-seryl-
aspartyl-lysyl-proline.12
IL-6 stimulates the
production of hepcidin in the hepatic cells, which
blocks absorption of iron in duodenum and
down-regulates ferroprotein expression, which in
turn prevents release of iron from total body
stores.13
In contrast, TNF-a and IL-6 inhibit eryth-
ropoietin production in the kidney by activating the
GATA-binding protein, GATA2, and nuclear factor
kB and also inhibits proliferation of bone marrow
erythroid progenitor cells.3,4,13
Some have used
erythropoietin receptor–stimulating agents or
intravenous iron to correct the anemia of heart
failure, but concern has arisen about the true
effectiveness of this approach and morbidity asso-
ciated with the therapy. The Reduction of Events
with Darbopoeitin Alfa in Heart Failure is a large-
scale, phase III, placebo-controlled, randomized,
morbidity and mortality clinical trial designed to
clarify these issues and will likely be finished
recruiting in 18 months.14
To unravel these complex interactions in cardi-
orenal anemia, Anil Agarwal, MD, Stuart Katz,
MD, and Ajay Singh, MD, have assembled a multi-
disciplinary team of experts in this field. In our
opinion, their multidisciplinary approach is essen-
tial to ensure that patients with cardiorenal anemia
stay in the pink of health.
Ragavendra R. Baliga, MD, MBA
Division of Cardiovascular Medicine
The Ohio State University
Columbus, OH, USA
James B. Young, MD
Division of Medicine and Lerner
College of Medicine, Cleveland Clinic
Cleveland, OH, USA
E-mail addresses:
Ragavendra.Baliga@osumc.edu (R.R. Baliga)
YOUNGJ@ccf.org (J.B. Young)
REFERENCES
1. Allen LA, Anstrom KJ, Horton JR, et al. Relationship
between anemia and health care costs in heart
failure. J Card Fail 2009;15(10):843–9.
2. Tanner H, Moschovitis G, Kuster GM, et al. The prev-
alence of anemia in chronic heart failure. Int J Cardiol
2002;86(1):115–21.
3. Anand IS. Anemia and chronic heart failure implica-
tions and treatment options. J Am Coll Cardiol 2008;
52(7):501–11.
4. Dec GW. Anemia and iron deficiency–new thera-
peutic targets in heart failure? N Engl J Med 2009;
361(25):2475–7.
5. Baliga RR, Young JB. ‘‘Stiff central arteries’’
syndrome: does a weak heart really stiff the kidney?
Heart Fail Clin 2008;4(4):ix–xii.
6. Ronco C, McCullough P, Anker SD, et al. Cardio-
renal syndromes: report from the consensus confer-
ence of the acute dialysis quality initiative. Eur Heart
J 2010;31(6):703–11.
7. Ronco C, Haapio M, House AA, et al. Cardiorenal
syndrome. J Am Coll Cardiol 2008;52(19):1527–39.
8. Nanas JN, Matsouka C, Karageorgopoulos D, et al.
Etiology of anemia in patients with advanced heart
failure. J Am Coll Cardiol 2006;48(12):2485–9.
9. Felker GM. Too much, too little, or just right?: untan-
gling endogenous erythropoietin in heart failure.
Circulation 2010; 121(2):191–3.
10. Opasich C, Cazzola M, Scelsi L, et al. Blunted eryth-
ropoietin production and defective iron supply for
erythropoiesis as major causes of anaemia in patients
with chronic heart failure. Eur Heart J 2005;26(21):
2232–7.
11. Ishani A, Weinhandl E, Zhao Z, et al. Angiotensin-
converting enzyme inhibitor as a risk factor for
the development of anemia, and the impact of
incident anemia on mortality in patients with left
ventricular dysfunction. J Am Coll Cardiol 2005;
45(3):391–9.
12. van der Meer P, Lipsic E, Westenbrink BD, et al. Levels
ofhematopoiesisinhibitorN-acetyl-seryl-aspartyl-lysyl-
proline partially explain the occurrence of anemia in
heart failure. Circulation 2005;112(12):1743–7.
13. Weiss G, Goodnough LT. Anemia of chronic disease.
N Engl J Med 2005;352(10):1011–23.
14. McMurray JJV, Anand IS, Diaz R, et al. Design of the
reduction of events with darbepoetin alfa in heart
failure (RED-HF): a phase III, anaemia correction,
morbidity-morality trial. Eur J Heart Fail 2009;11:
795–801.
Editorialxvi](https://image.slidesharecdn.com/cardiorenalanemiahfclinics-160204111316/85/Cardio-renalanemiahf-clinics-6-320.jpg)