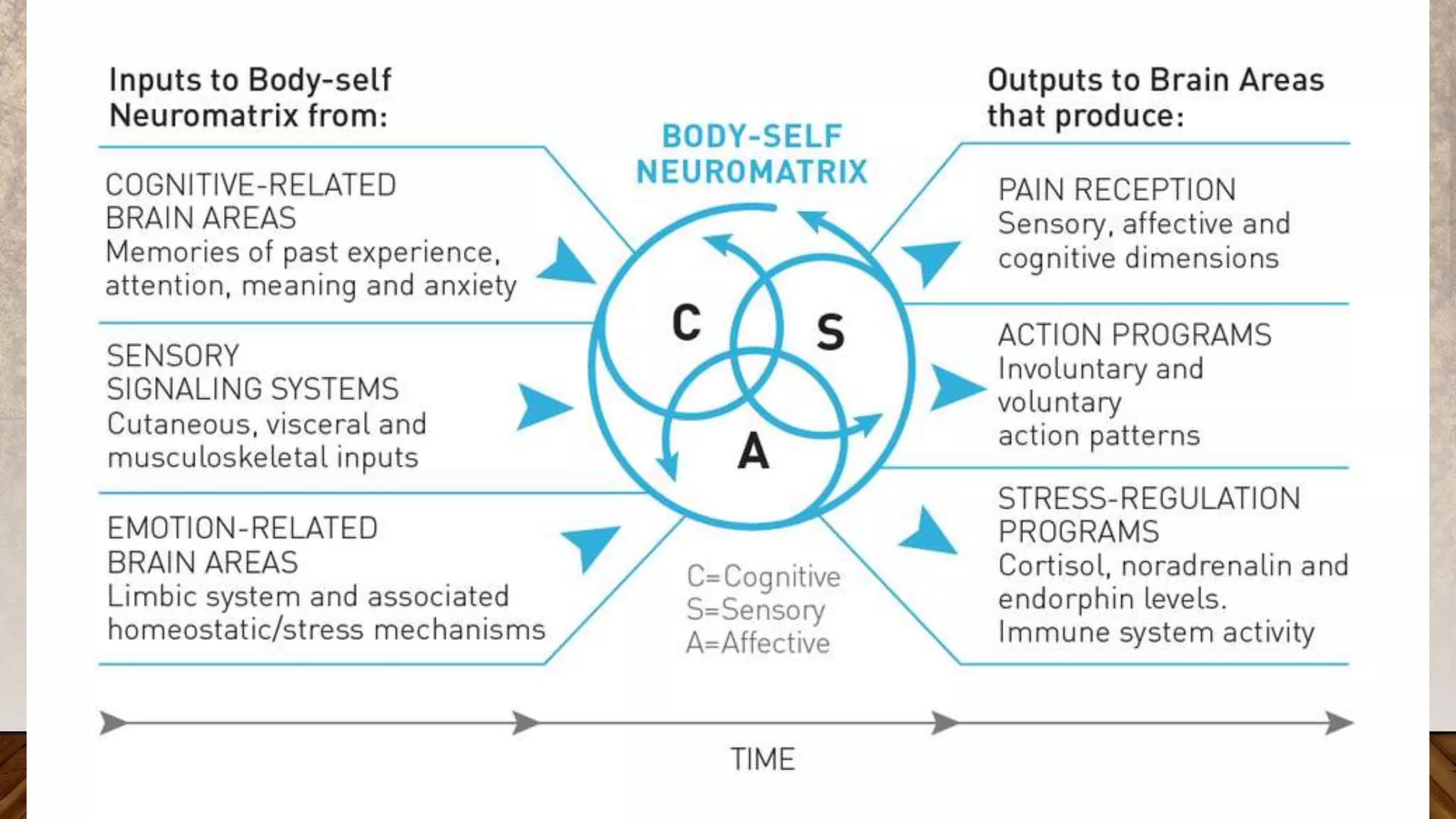
1. The document discusses the pain pathway and summarizes several theories of pain including the intensity theory, Cartesian dualism theory, specificity theory, pattern theory, gate control theory, neuromatrix model, and biopsychosocial theory.
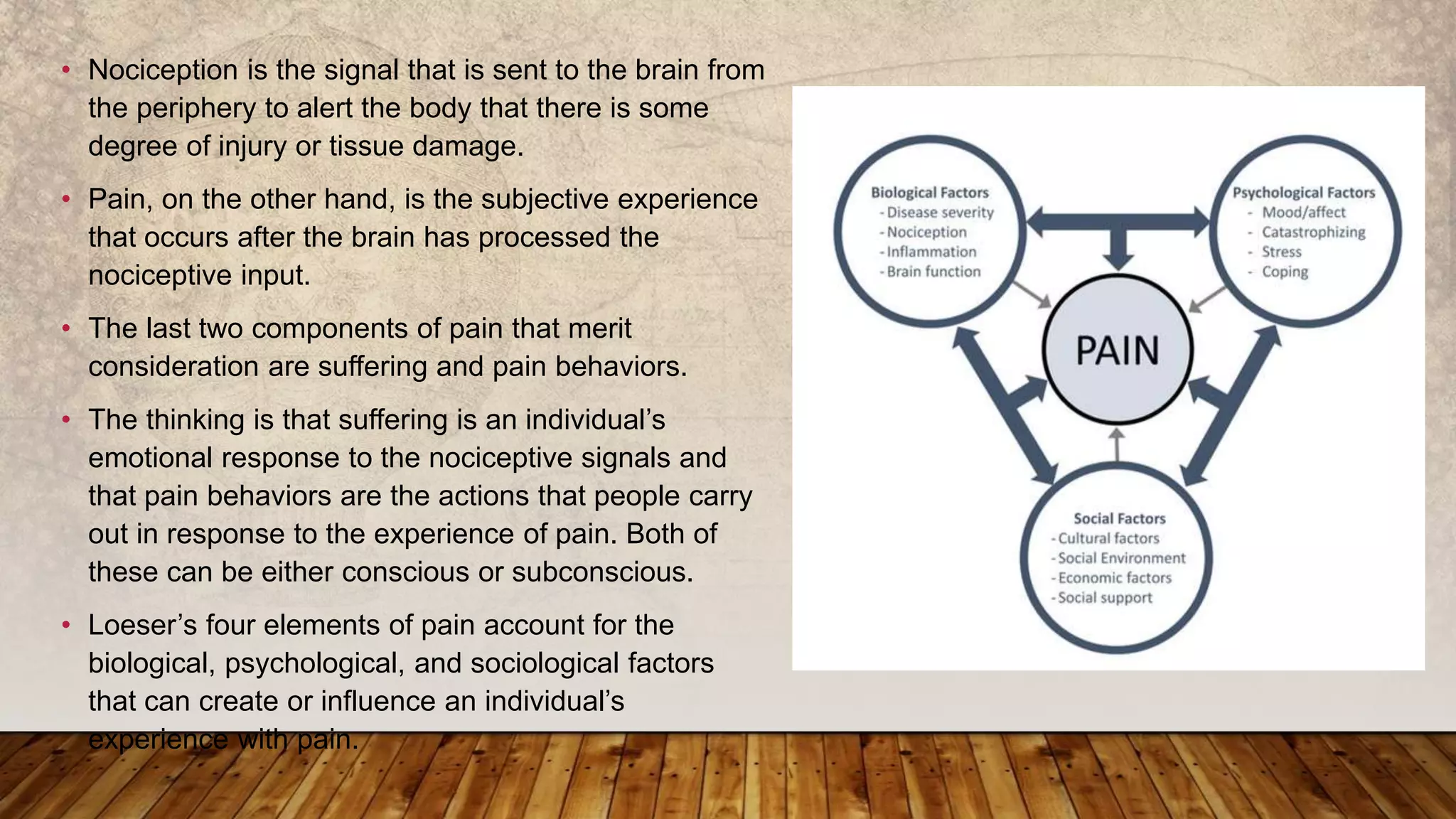
2. It provides definitions of pain from sources like the IASP and discusses classifications of pain such as by location, duration, and underlying pathology.
3. The neuroanatomy and physiology of pain are described including the transduction, transmission, and perception of pain signals in the nervous system.
































































![28. Ide M. The differential diagnosis of sensitive teeth. Dent Update 1998; 25: 462- 66.
29. Sessile BJ. Neurophysiology of Oro facial Pain. In: Curro FA, editor. Dent Clin North Am 1987;
31(4):595-613.
30. Okeson JP. The Psychology of Pain. In: Okeson JP, editor. Bell‘s Orofacial Pains.5th ed. USA.
Quintessence Pub Co;1995. p. 93-102.
31. Melzack R. Neurophysiological Foundations of pain. In: Sternbach RA, editor. The Psychology of
pain. 2nd ed. New York. Raven Press; 1986. p. 1-25.
32. Cascella M, Thompson NS, Muzio MR, Forte CA, Cuomo A. The underestimated role of
psychological and rehabilitation approaches for management of cancer pain. A brief commentary.
Recenti Prog Med. 2016 Aug;107(8):418-21. [PubMed: 27571557]
33. Spofford CM, Brennan TJ. Gene expression in skin, muscle, and dorsal root ganglion after plantar
incision in the rat. Anesthesiology. 2012 Jul;117(1):161-72. [PMC free article: PMC3389501] [PubMed:
22617252]
34. Cascella M, Muzio MR. Pain insensitivity in a child with a de novo interstitial deletion of the long arm
of the chromosome 4: Case report. Rev Chil Pediatr. 2017 Jun;88(3):411-416. [PubMed: 28737203]](https://image.slidesharecdn.com/painpathway-210522140031/75/Pain-pathway-69-2048.jpg)
![35. Moayedi M, Davis KD. Theories of pain: from specificity to gate control. J. Neurophysiol. 2013
Jan;109(1):5-12. [PubMed: 23034364] 36. Miceli L, Bednarova R, Rizzardo A, Cuomo A, Riccardi I,
Vetrugno L, Bove T, Cascella M. Opioids prescriptions in pain therapy and risk of addiction: a one-
year survey in Italy. Analysis of national opioids database. Ann. Ist. Super. Sanita. 2018 Oct-
Dec;54(4):370-374. [PubMed: 30575575]
36. Chen J. History of pain theories. Neurosci Bull. 2011 Oct;27(5):343-50. [PMC free article:
PMC5560314] [PubMed: 21934730]
37. Bernhard Naunyn (1839-1925), clinician, teacher, scientist. JAMA. 1969 May 19;208(7):1182-3.
[PubMed: 4894191]
38. Cuomo A, Bimonte S, Forte CA, Botti G, Cascella M. Multimodal approaches and tailored therapies
for pain management: the trolley analgesic model. J Pain Res. 2019;12:711-714. [PMC free article:
PMC6388734] [PubMed: 30863143]](https://image.slidesharecdn.com/painpathway-210522140031/75/Pain-pathway-70-2048.jpg)
