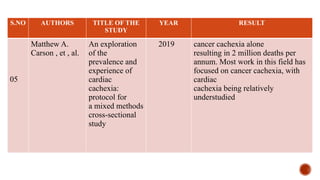
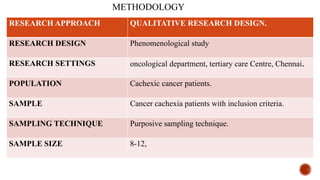
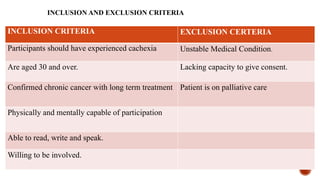
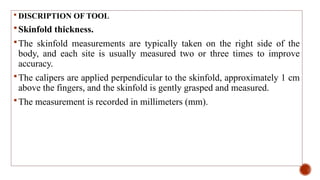
This document presents a research proposal by Deepika R. focusing on the experiences of cachexia among cancer survivors at a tertiary care center in Chennai. The study aims to explore the severity of cachexia, the lived experiences of survivors, coping mechanisms, and their support needs, highlighting the significant impact of cachexia on quality of life. It emphasizes the need for patient-centered strategies and comprehensive care interventions to address the physical, emotional, and social challenges faced by these individuals.