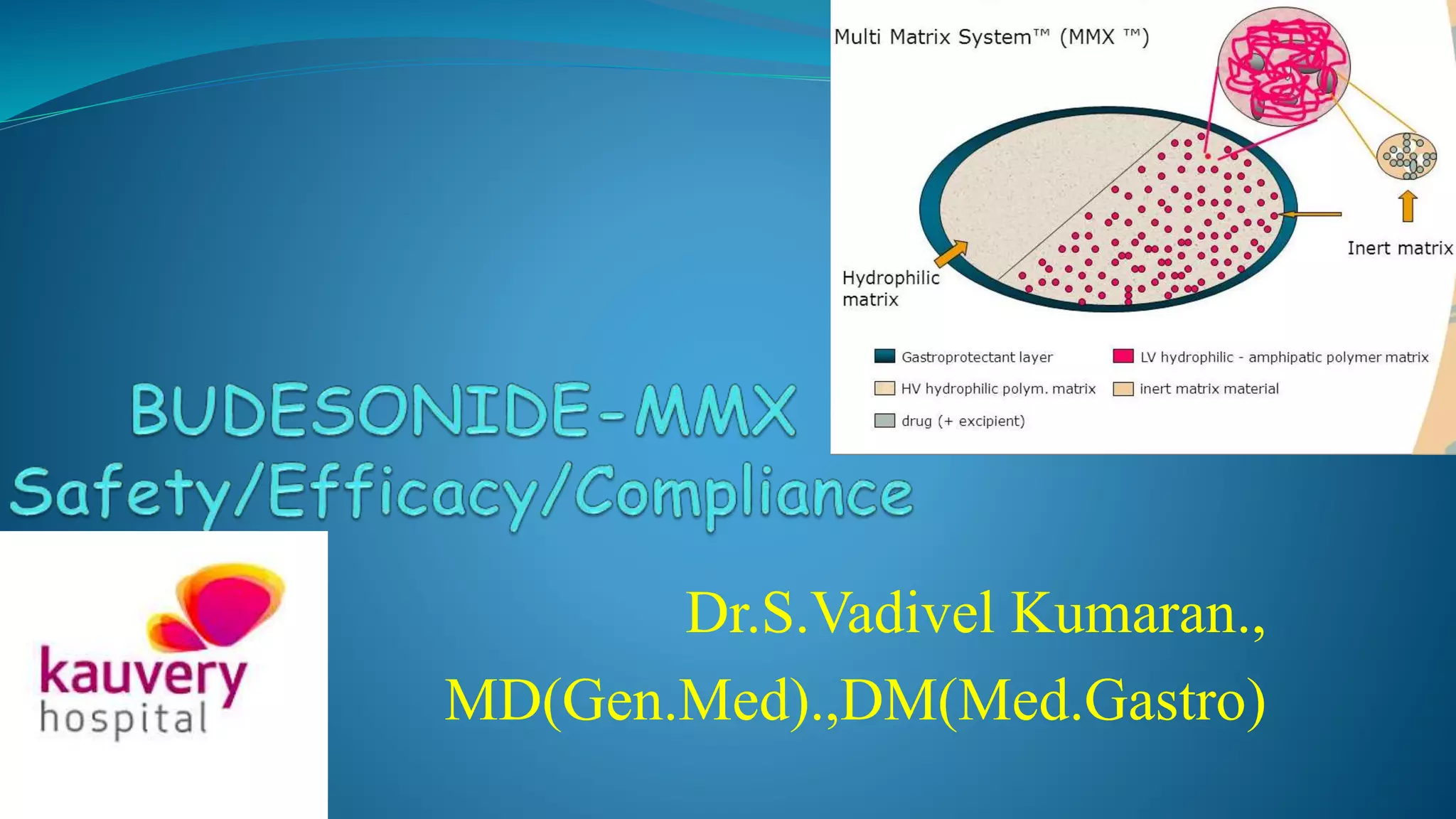
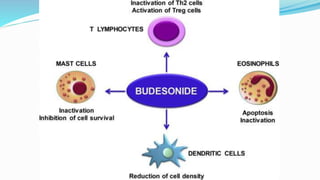
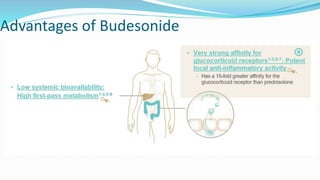
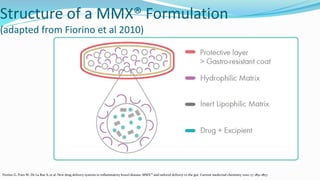
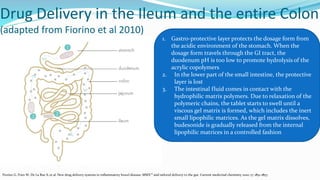
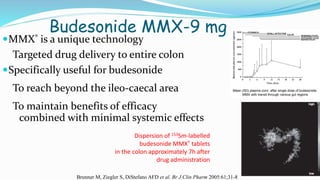
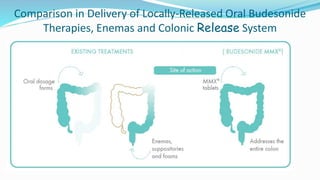
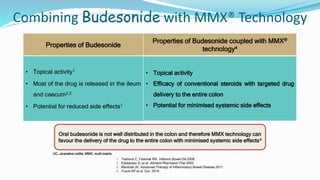
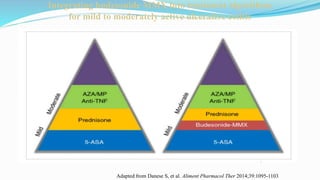
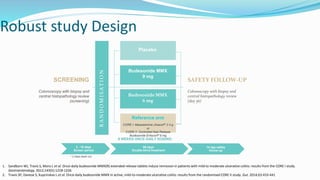
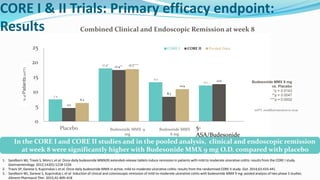
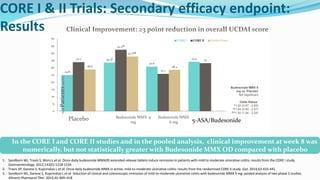
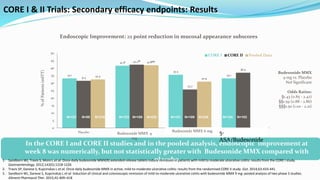
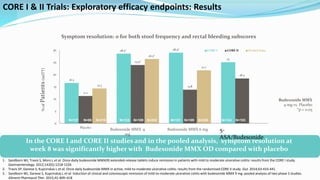
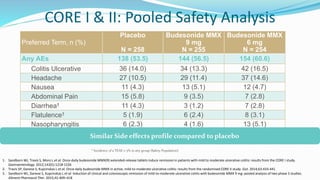
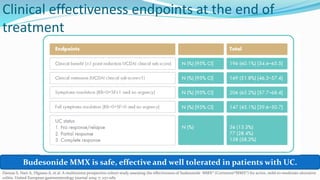
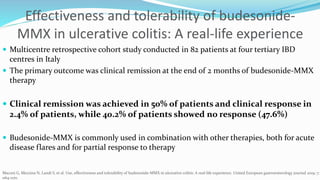
Budesonide MMX is a novel formulation that delivers the corticosteroid budesonide directly to the colon for the treatment of mild-to-moderate ulcerative colitis. Two pivotal trials, CORE I and CORE II, found that the 9mg dose of Budesonide MMX achieved significantly higher rates of combined clinical and endoscopic remission at 8 weeks compared to placebo. Budesonide MMX was also found to be well-tolerated with a safety profile similar to placebo. The targeted delivery of budesonide to the colon provides efficacy comparable to conventional corticosteroids but with potentially fewer systemic side effects.