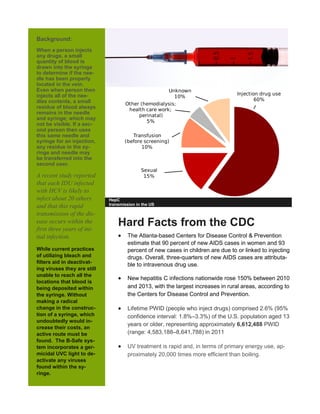
The B-Safe system aims to prevent the spread of viruses like HIV and hepatitis C among intravenous drug users. It consists of a portable case containing a syringe that is sanitized using ultraviolet-C light. When a user closes the case for five minutes after injecting, the light deactivates viruses in the syringe that may be transmitted if the syringe is reused. The system seeks to address the risks of blood residue remaining in reused syringes. Current prototypes need further development and testing before the cases can be distributed to active drug users for real-world testing of effectiveness and usability.