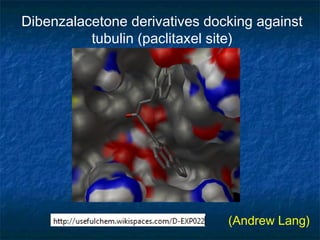
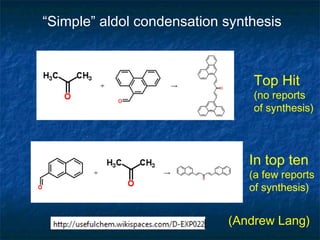
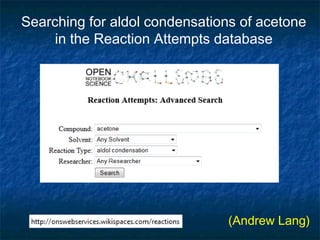
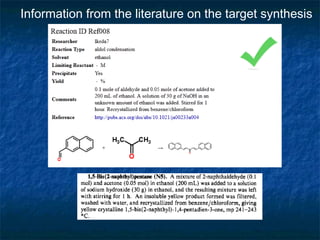
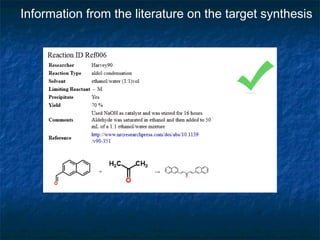
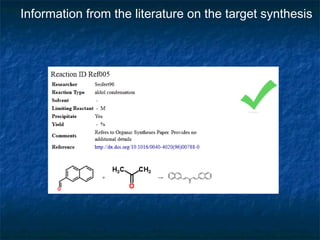
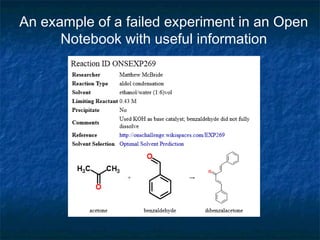
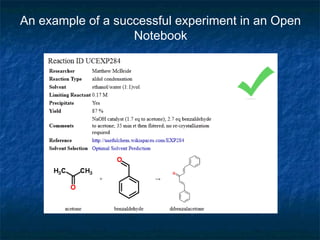
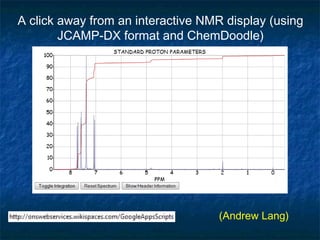
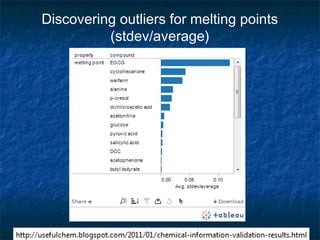
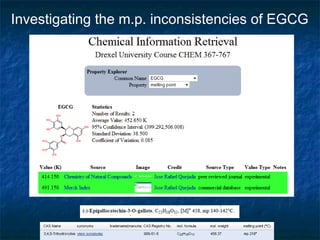
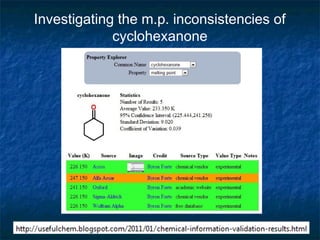
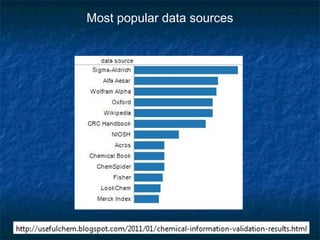
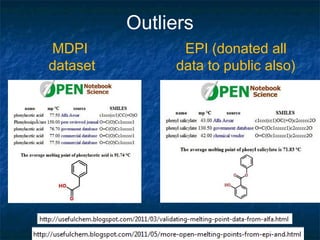
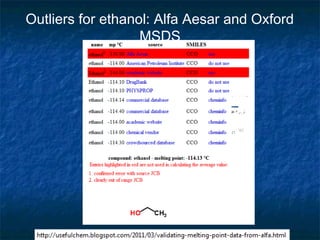
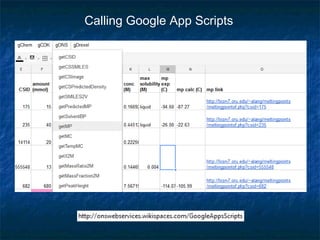
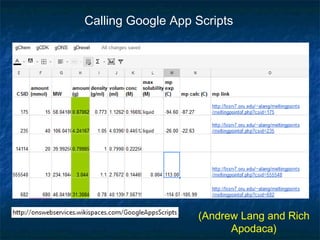
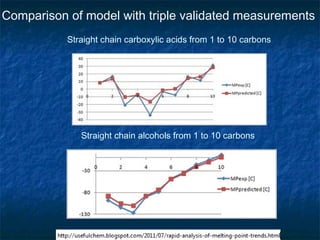
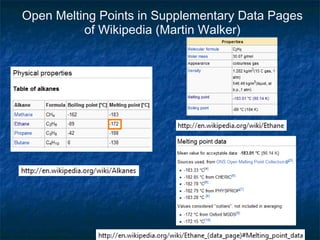
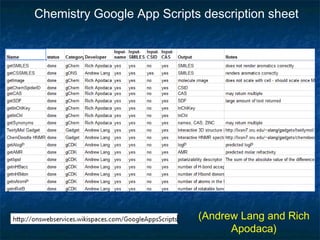
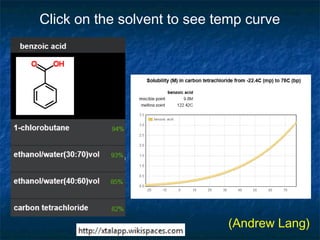
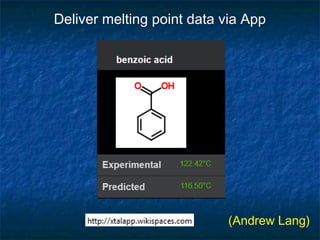
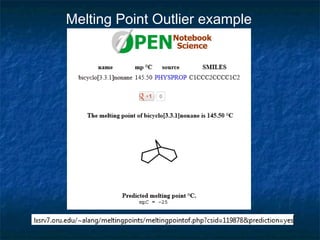
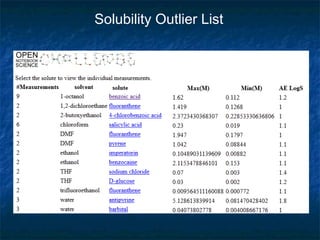
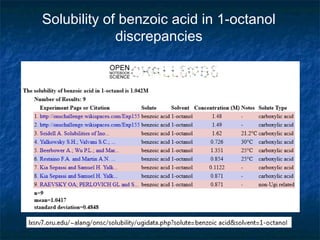
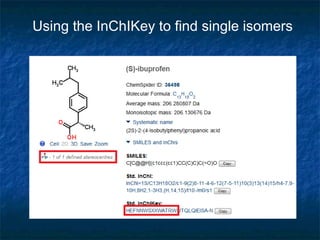
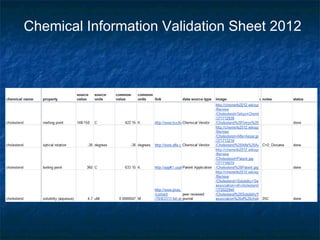
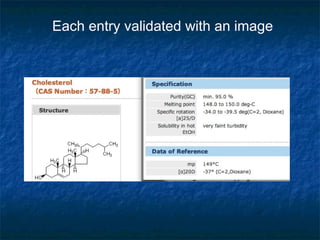
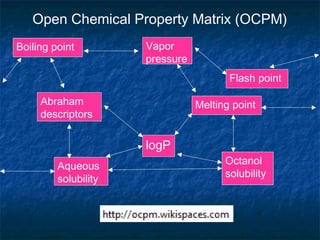
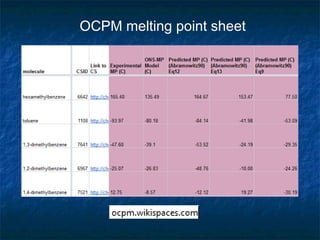
The document discusses the principles and applications of open notebook science in the field of chemistry, emphasizing transparency and accessibility of research data. It highlights various techniques for synthesizing compounds, validating chemical information, and utilizing open data for predicting melting points and selecting solvents. The overarching goal is to improve the efficiency of scientific research through greater openness and collaboration among chemists.