Embed presentation
Download to read offline
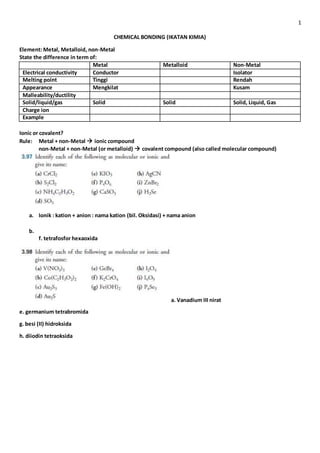






Metals can conduct electricity and have high melting points, while non-metals exist in solid, liquid, and gas phases with low melting points. Compounds formed between metals and non-metals are ionic, with the metal transferring electrons to the non-metal. Compounds formed between non-metals or metals and metalloids are covalent, with shared pairs of electrons. Bond length and bond energy are also influenced by the types of elements involved in a chemical bond.






