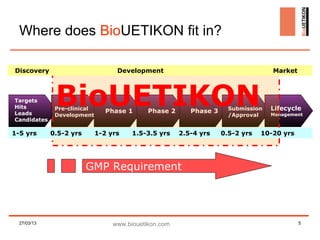
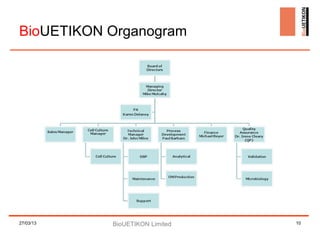
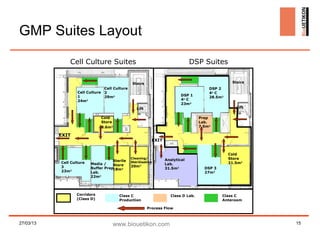
Biouetikon Limited, founded in Dublin, offers high-quality bioprocess development and GMP production services, targeting biotech companies in Europe and the US. The company aims to improve its brand awareness and credibility as a contract manufacturing organization (CMO) while maintaining compliance with international quality standards. Challenges include market visibility and establishing reputation among established competitors in a conservative industry.