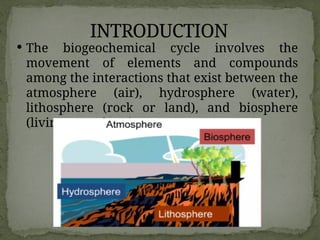
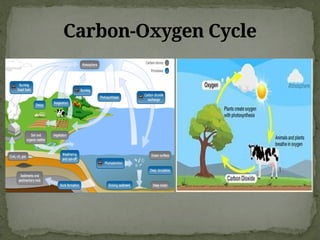
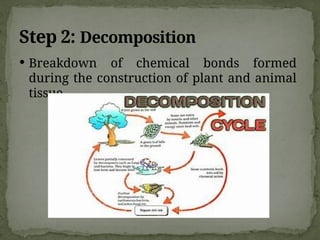
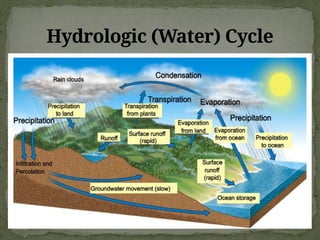
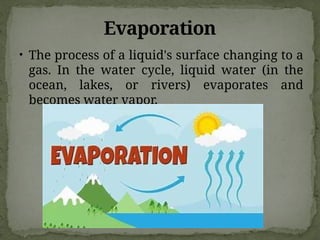
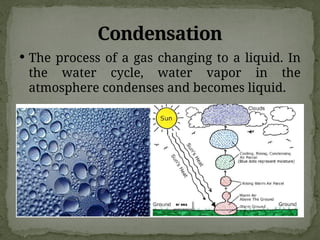
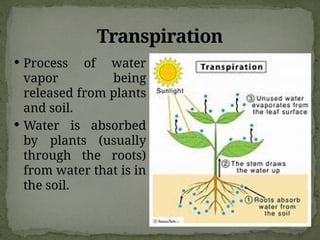
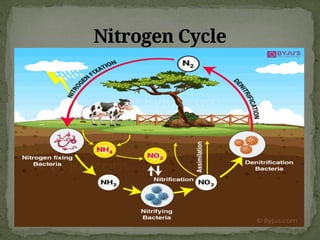
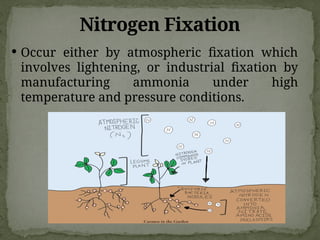
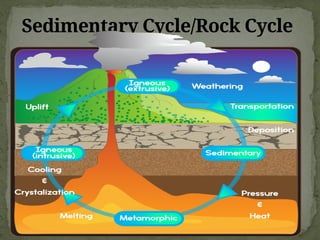
The document discusses biogeochemical cycles, which include the carbon-oxygen cycle, water cycle, nitrogen cycle, and sedimentary cycle. It details the processes within each cycle, such as photosynthesis and decomposition in the carbon cycle, evaporation and precipitation in the water cycle, and nitrogen fixation and denitrification in the nitrogen cycle. Additionally, the document outlines the formation of different rock types and the processes involved in the sedimentary cycle.