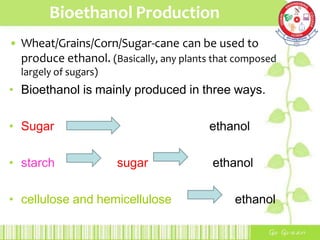
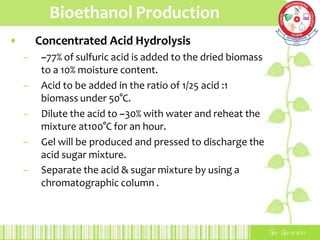
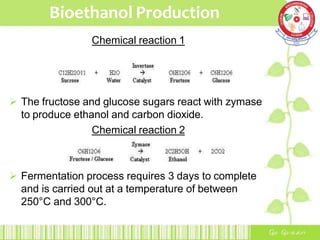
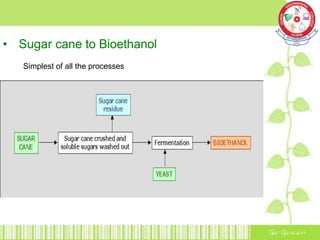
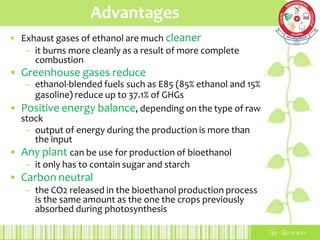
This document summarizes the production of bioethanol from sugar cane waste. It discusses that bioethanol is an alcohol made by fermenting carbohydrates from plants like sugarcane and corn. There are three main methods of bioethanol production: sugar ethanol from sugarcane, starch ethanol from grains, and cellulosic ethanol from plant waste. The production process involves hydrolysis to break down biomass into sugars, fermentation of the sugars into ethanol using yeast, and fractional distillation to separate ethanol from water. Bioethanol has advantages as a cleaner-burning, renewable fuel but also has disadvantages related to energy efficiency, land use, and food competition. Future development aims to make bioethanol