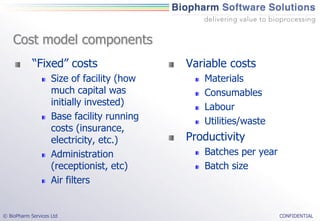
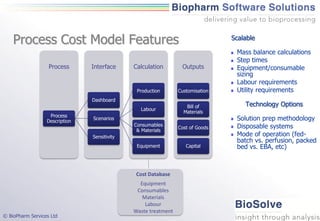
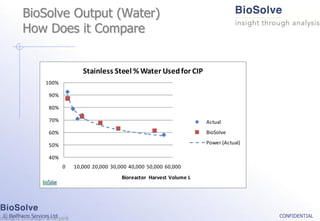
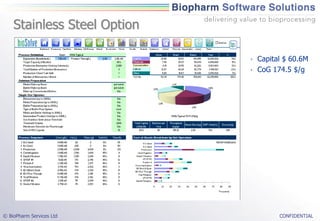
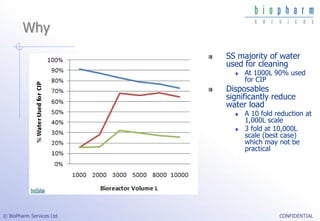
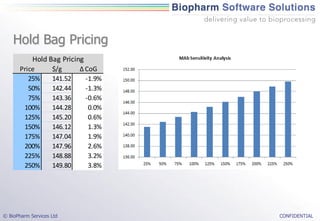
The document discusses the evaluation of single-use technologies in biopharmaceutical manufacturing, highlighting the importance of cost modeling for decision-making and efficiency. It emphasizes the rising manufacturing costs, driven by increasing development expenses, and explores factors influencing cost structures, including fixed and variable costs. Additionally, the content outlines the benefits of process modeling to improve cost-effectiveness and streamline production using single-use systems versus traditional stainless steel vessels.